Draw A Sodium Atom
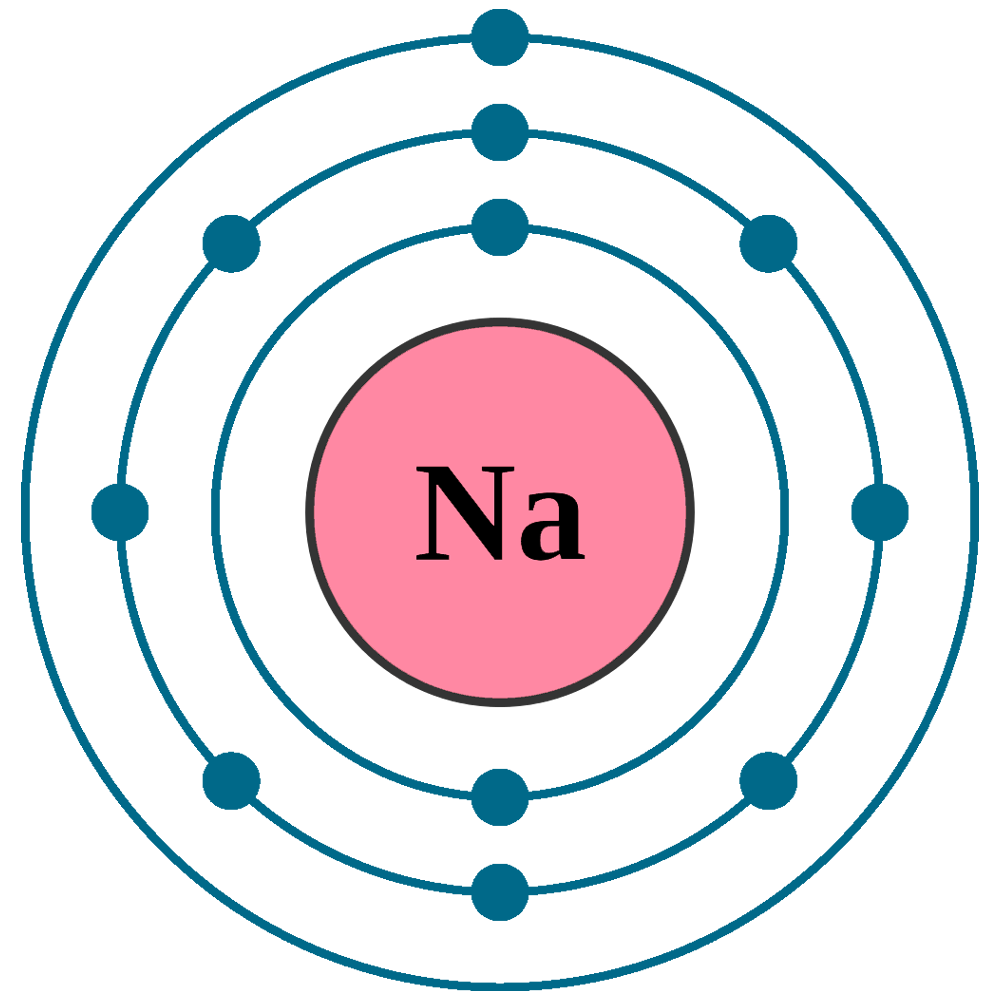
Draw A Sodium Atom - Web the sodium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, and the remaining one electron in the 3s orbital. It has a total of 11 11 protons and 11 11 electrons. When you learn about atoms in a chemistry class, you’ll learn a much more sophisticated and accurate model of electron arrangement. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. Web in the diagram above, we see a neutral atom of sodium, na, losing an electron. The atomic number of an element is equal to the number of protons and electrons in that element. Its outer shell will then have no electrons. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. Web updated on november 05, 2019. Web what happens when a sodium atom becomes a sodium ion? It is as though the outer shell has vanished. For the na+ structure use the periodic table to find the total number of valence. We draw atomic structures for any element with the help of atomic number they have. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Sodium usually forms ionic. Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2. When you learn about atoms in a chemistry class, you’ll learn a much more sophisticated and accurate model of electron arrangement. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. Web the sodium atom. Web the sodium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, and the remaining one electron in the 3s orbital. The electronic configuration of sodium is: A sodium atom has 1 electron in its outer shell. In almost all cases, chemical bonds are formed by interactions. Similarly, neon has a complete outer 2n shell containing eight electrons. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Web complete step by step answer: It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. It is in group 1 of the periodic. Additionally, sodium is found in group one, which means it has one valence electron. Web • the atomic mass of sodium is 22.989. Atomic number of sodium is 11. Web sodium is a classified alkali metal element and its symbol is na. Similarly, neon has a complete outer 2n shell containing eight electrons. Web the sodium atom (na) is commonly used for examples and practice problems in chemistry. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Web as shown, helium. Web the sodium orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, and the remaining one electron in the 3s orbital. A sodium atom has 1 electron in its outer shell. Web what happens when a sodium atom becomes a sodium ion? Web complete step by step. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. Web draw a lewis electron dot diagram for an atom or a monatomic ion. It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. #1 write protons, neutrons, and electrons of sodium atom #2 draw nucleus. Sodium has 2 electrons in its first shell, 8 in its second and 1 in its third. Web for atoms with more than two protons and two electrons, a few rules need to be introduced to diagram them in a way that represents their chemical properties. When you learn about atoms in a chemistry class, you’ll learn a much more. It is as though the outer shell has vanished. Example \(\pageindex{2}\) draw the bohr's model for sodium (na). Web the bohr model of sodium (na) is drawn with three electron shells, the first shell contains 2. Sodium is an atom in the periodic table with atomic number 11 11 and mass number 23 23. Web sodium is a chemical element; Web sodium is a chemical element; Similarly, neon has a complete outer 2n shell containing eight electrons. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Web draw a lewis electron dot diagram for an atom or a monatomic ion. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Even a minute amount of water can create this type of reaction. #1 write protons, neutrons, and electrons of sodium atom When you learn about atoms in a chemistry class, you’ll learn a much more sophisticated and accurate model of electron arrangement. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted zigzag line. Web • the atomic mass of sodium is 22.989. For that, we have electron shell diagrams. Web updated on november 05, 2019. Web complete step by step answer: To draw the bohr model of sodium we must first find out. Sodium is an atom in the periodic table with atomic number 11 11 and mass number 23 23.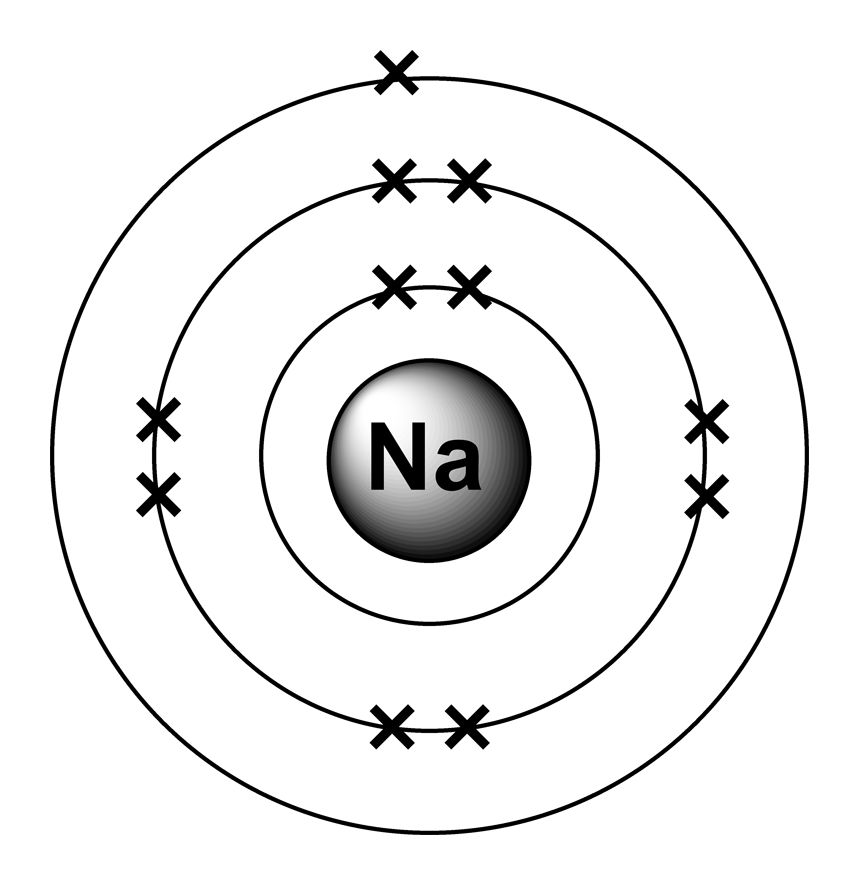
Sodium Table of Elements by Shrenil Sharma
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
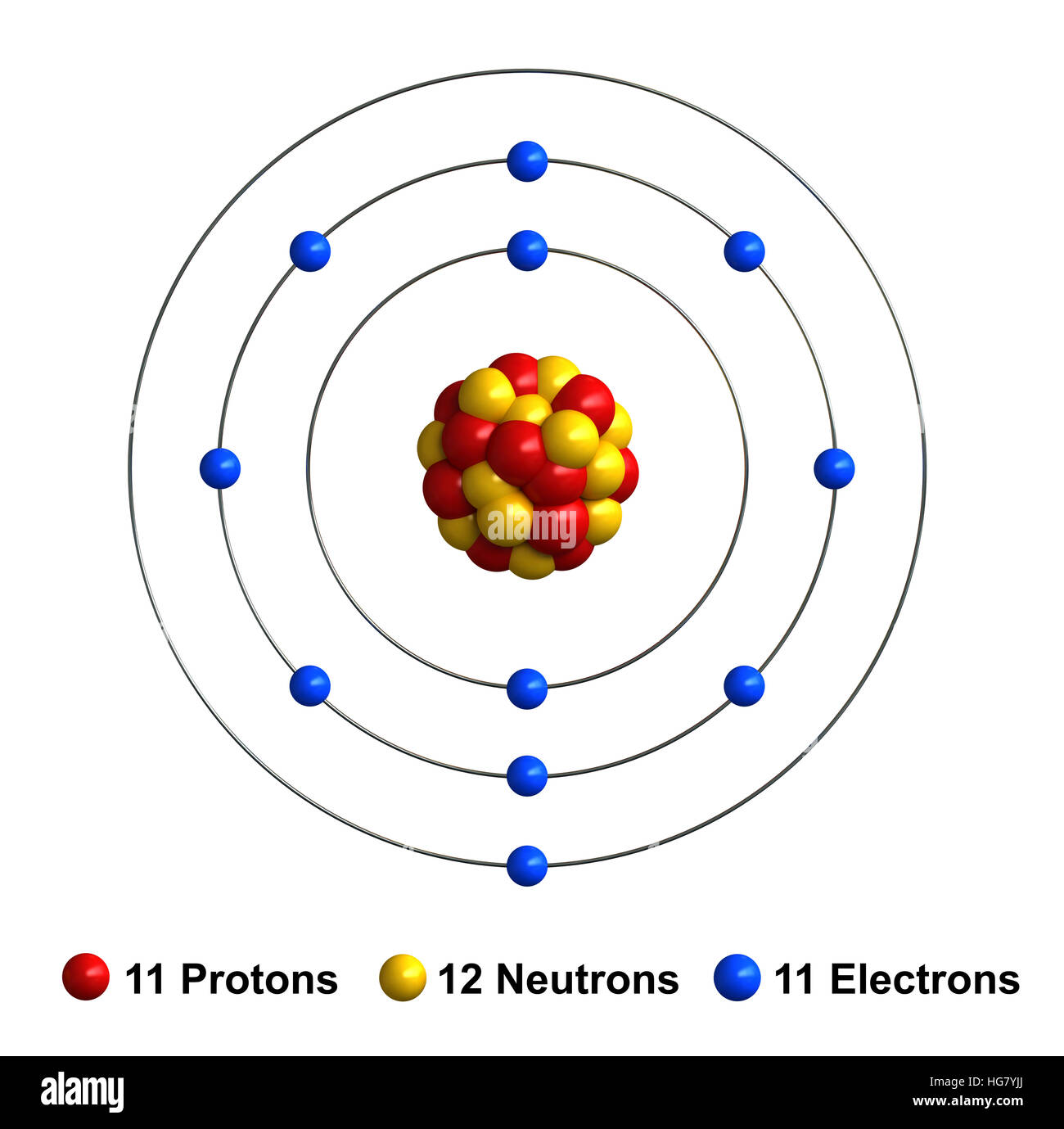
3d render of atom structure of sodium isolated over white background

Sodium atom polizworth
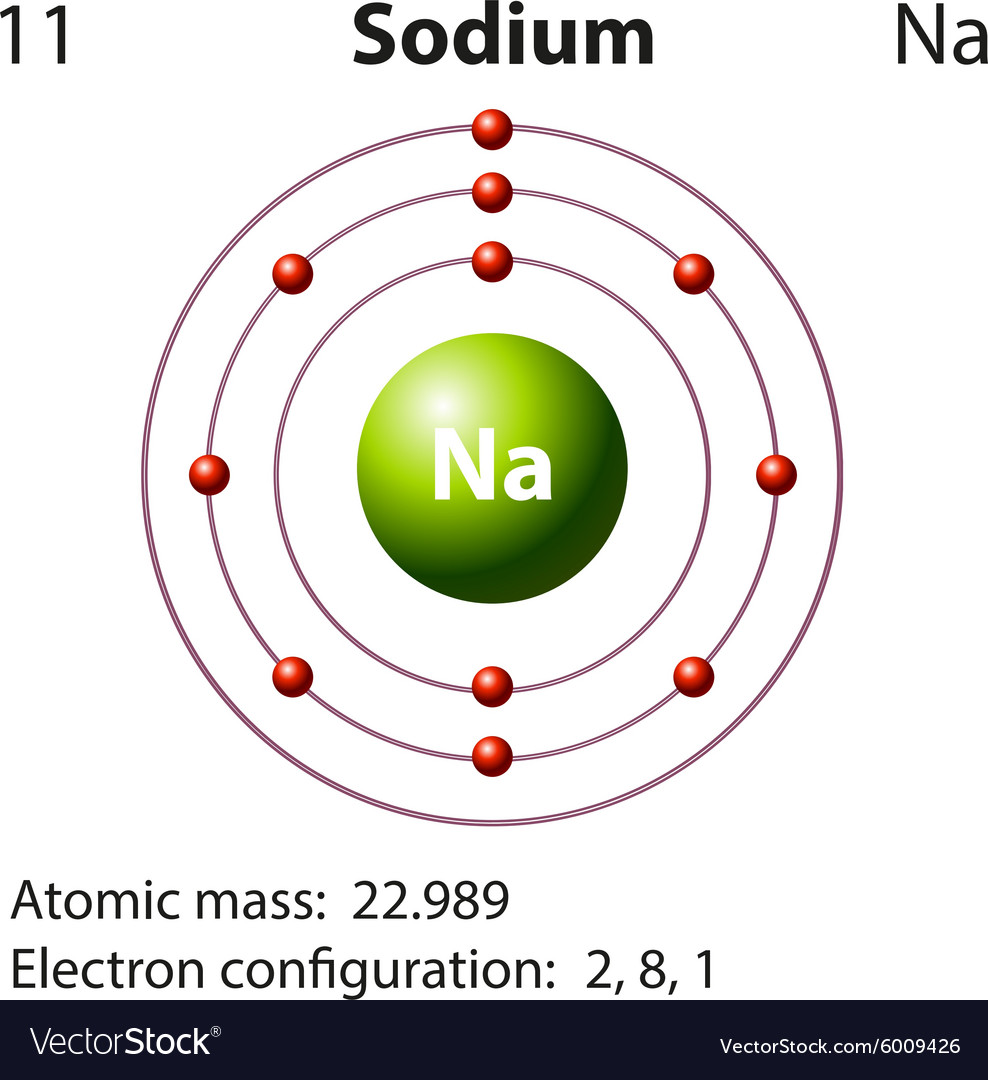
Diagram representation of the element sodium Vector Image

FileElectron shell 011 sodium.png Wikimedia Commons
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
Sodium Na (Element 11) of Periodic Table NewtonDesk

Atom sodium Royalty Free Vector Image VectorStock
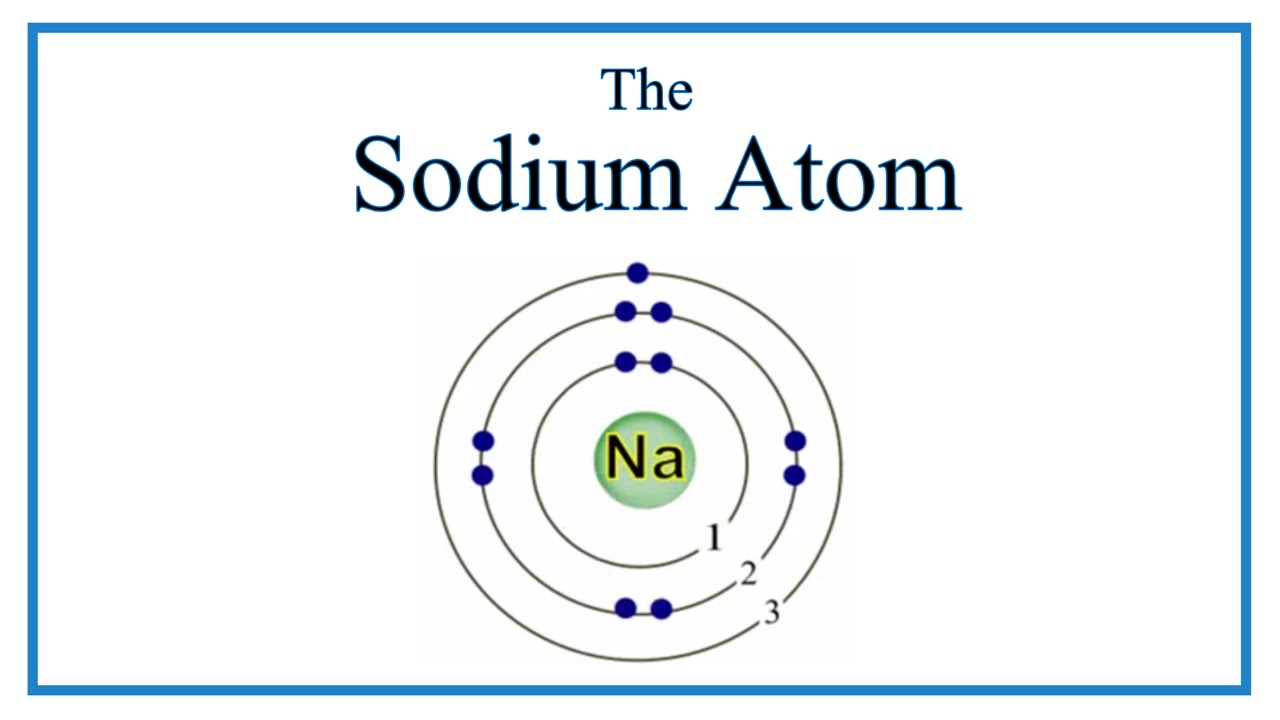
Atomic Structure of the Sodium Atom (Na) YouTube
The Result Is That The Sodium Ion, Na + , Has 11 Protons, But Only 10 Electrons.
In Contrast, Chlorine And Sodium Have Seven And One Electrons In Their Outer Shells, Respectively.
In This Video We'll Look At The Atomic Structure, Valence Electrons,.
A Bhor's Model Can Be Used To Diagram The Location Of Electrons In Each Energy Shell For An Atom.
Related Post: