Draw All Resonance Structures For The Ozone Molecule O3
Draw All Resonance Structures For The Ozone Molecule O3 - Do not show ion charges in your drawings. Remember, valence electrons are those in the outermost principal energy level.for example: Solution verified by toppr (i) resonating structure of ozone ( o 3 ): The two possible lewis structures that can be drawn for ozone are Web draw the most valid lewis structure for ozone (o3): Draw the lewis structure for the ozone (03) molecule. Such is the case for ozone (\(\ce{o3}\)), an allotrope of oxygen with a v. Web in this post, we will be drawing the lewis structure of ozone, o 3. It is not a ring, although that might be tempting. If there are equivalent resonance structures, draw all of them. Web the lewis structure of ozone has: 10 similar questions q 1 resonating structures in ozone: Web figure 5.3.4 the resonance structure of ozone involves a molecular orbital extending all three oxygen atoms. Similar molecular orbitals are found in every resonance structure. But as we draw lewis structures and we follow those rules, it's a little bit hard to think. Solution verified by toppr (i) resonating structure of ozone ( o 3 ): For molecules with multiple resonance structures, draw all possible resonance structures (in the same drawing window): The two possible lewis structures that can be drawn for ozone are After drawing the lewis structure of nh 3, you can decide shape of the o 3 molecule. Draw one. Draw one structure per sketcher box, and separate added sketcher boxes with the ↔ symbol. Web in this post, we will be drawing the lewis structure of ozone, o 3. Similar molecular orbitals are found in every resonance structure. Web the lewis structure of ozone has: Press and hold explanation check. Web the lewis structure of ozone has: (figure 1) (ii) resonating structure of n o 3 −: I also go over the resonance, hybridization, shape and bond angle. Such is the case for ozone (\(\ce{o3}\)), an allotrope of oxygen with a v. For molecules with multiple resonance structures, draw all possible resonance structures (in the same drawing window): Each step of drawing the lewis structure of o 3 is explained in detail in this tutorial. (figure 2) was this answer helpful? But as we draw lewis structures and we follow those rules, it's a little bit hard to think that way. (figure 1) (ii) resonating structure of n o 3 −: Web chemistry bond parameters question draw the. Web science chemistry chemistry questions and answers draw all resonance structures for the ozone molecule, o3. For molecules with multiple resonance structures, draw all possible resonance structures (in the same drawing window): But as we draw lewis structures and we follow those rules, it's a little bit hard to think that way. Web figure 5.3.4 the resonance structure of ozone. Solution verified by toppr (i) resonating structure of ozone ( o 3 ): It's an average of them. This structure predicts that the two bonds are different lengths and strengths, because double bonds are shorter and stronger than single bonds. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons. If there are equivalent resonance structures, draw all of them. But as we draw lewis structures and we follow those rules, it's a little bit hard to think that way. * three oxygen atoms in a row. After drawing the lewis structure of nh 3, you can decide shape of the o 3 molecule. (figure 1) (ii) resonating structure of. Web the lewis structure of ozone has: Press and hold explanation check. Web for example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the molecule. The two possible lewis structures that can be drawn for ozone are Solution verified by toppr (i) resonating structure of ozone ( o 3 ): Web science chemistry chemistry questions and answers draw all resonance structures for the ozone molecule, o3. * three oxygen atoms in a row. Remember, valence electrons are those in the outermost principal energy level.for example: Both bonds in ozone have a bond order of 1.5 and are the same length, which is longer than a double bond (because the double. The molecule’s 1/2 bond order, which indicates the number of bonds between two atoms, also supports the notion that the hybrid structure incorporates the ozone molecule’s two principal resonance configurations. Web 1 bond + 3 lone pairs = negatively charged oxygen atom 3 bonds and 1 lone pair = positively charged oxygen so, in summary, if a professors asks you how many resonance structures can be drawn for ozone o 3, your answer should be a (confident) two. The two possible lewis structures that can be drawn for ozone are Remember, valence electrons are those in the outermost principal energy level.for example: This problem has been solved! After drawing the lewis structure of nh 3, you can decide shape of the o 3 molecule. Draw the lewis structure for the ozone (03) molecule. Web draw the most valid lewis structure for ozone (o3): (figure 1) (ii) resonating structure of n o 3 −: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (figure 2) was this answer helpful? Web in this post, we will be drawing the lewis structure of ozone, o 3. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons in the molecule. Draw lewis structure(s) for the ozone molecule (o3). Similar molecular orbitals are found in every resonance structure. Ozone and the ozonolysis of alkenes how many resonance structures can be drawn for.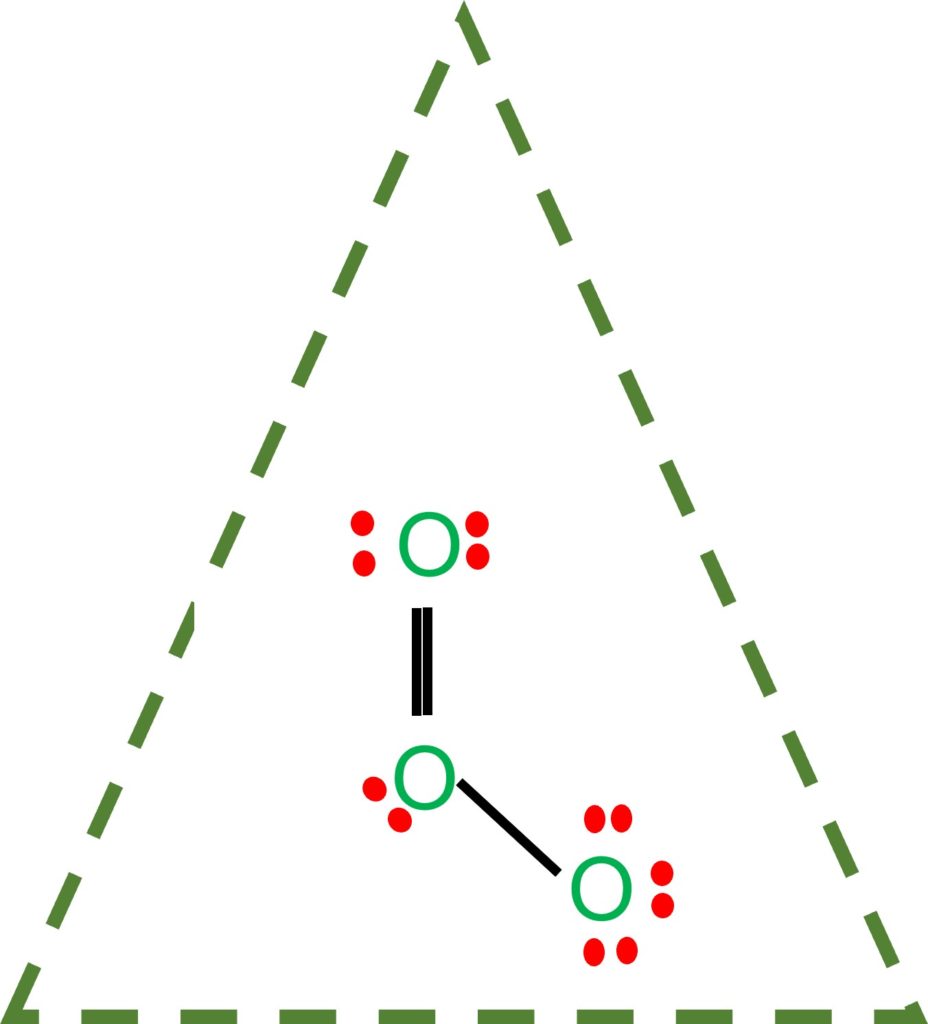
O3 Lewis Structure Step By Step Drawing What's Insight
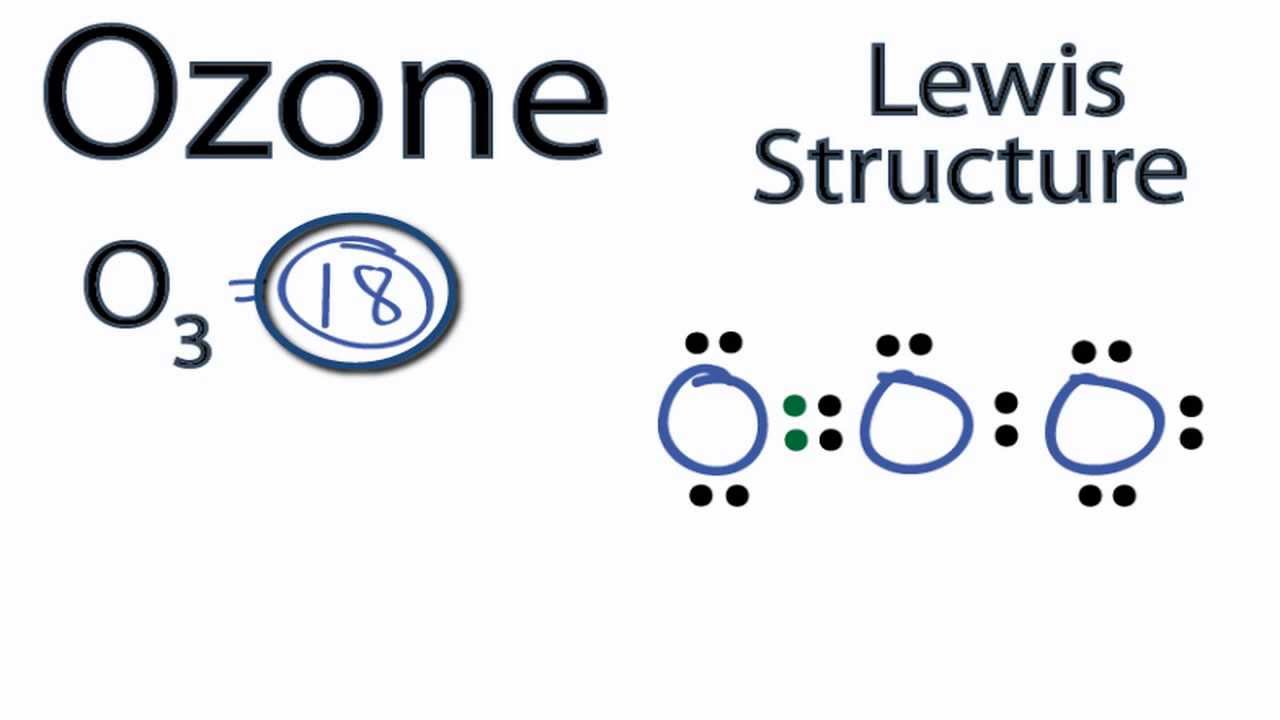
Ozone Lewis Structure How to Draw the Lewis Structure for Ozone YouTube

Resonance Structures of O3, Ozone YouTube

O3 Resonance Structures (Ozone) YouTube
Resonance Presentation Chemistry
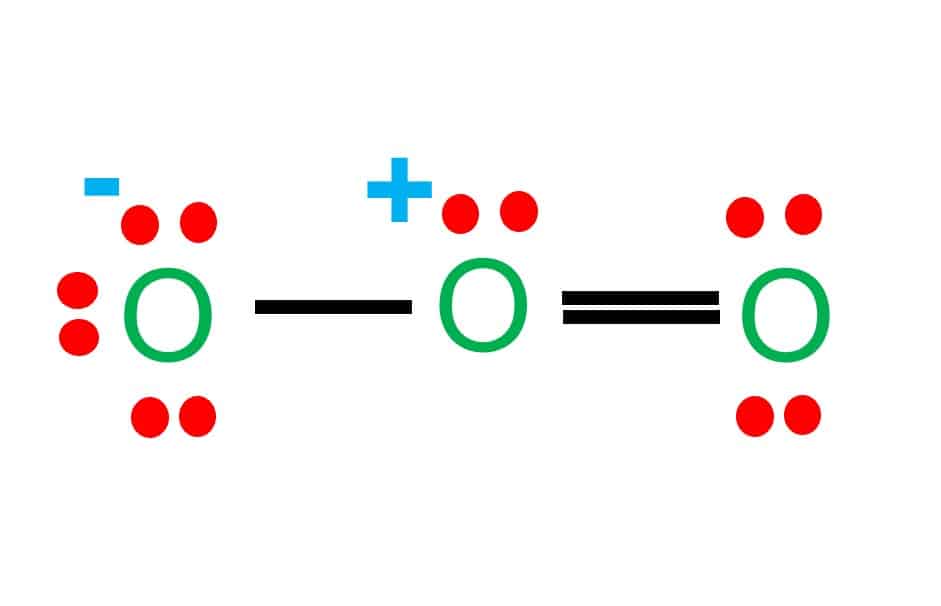
O3 Lewis Structure Step By Step Drawing What's Insight

O3 Lewis Structure How to Draw the Dot Structure for O3 YouTube

O3 Lewis Structure (Ozone) Chemistry, Ozone, Lewis

12+ O3 Lewis Structure Robhosking Diagram

Resonance Structures Easy Hard Science
If We Draw A Lewis Structure For O 3 (Ozone), We Get This:
* Two Resonance Structures * A Lone Pair On The Central Oxygen Atom * Ax2E Vsepr.
Web Science Chemistry Chemistry Questions And Answers Draw All Resonance Structures For The Ozone Molecule, O3.
Draw One Structure Per Sketcher Box, And Separate Added Sketcher Boxes With The ↔ Symbol.
Related Post:
.PNG)