Draw An Mo Energy Diagram For Co
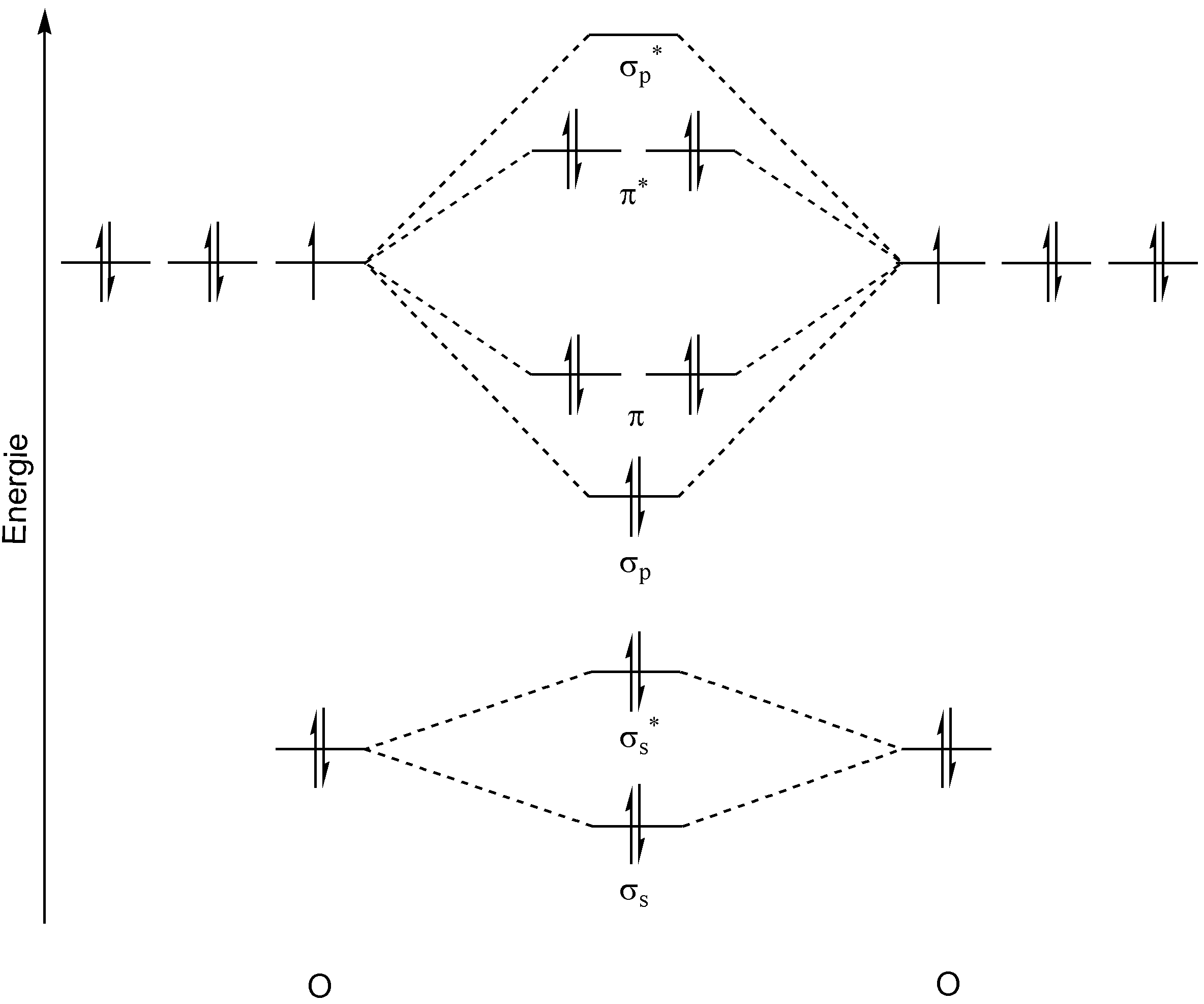
Draw An Mo Energy Diagram For Co - A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. Not all targets will be filled. Carbon has the electron configuration 1s² 2s² 2p², and oxygen has the electron configuration 1s². The carbon in the co molecule is the more reactive end, and thus co prefers to bind with the carbon to a metal, and not with oxygen. Web the bigger the difference in electronegativity/ao energy, the smaller the splitting and the more the bonding mo looks like the ao of the electronegative atom. Identify the homo and explain why co bonds to metal ions through the carbon atom rather than through the oxygen atom. Sometimes, we may be interested in only the molecular orbital energy levels themselves, and not where they came from. This diagram helps visualize the arrangement of the molecular orbitals and their relative energies, providing valuable insights into the stability and reactivity of the molecule. For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Web draw an mo energy diagram for co. There are 3 steps to solve this one. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. Web draw an mo energy diagram for co. When the difference is really big, the bond becomes completely ionic, and the bonding mo basically is the lower energy ao. Draw a molecular. That mo is closer in energy to both the c 2s and 2p than the contributing o atomic orbitals. Mo theory • mo diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. When you look at an mo diagram, you can see what aos are included by checking the outside of the. This diagram helps visualize the arrangement of the molecular orbitals and their relative energies, providing valuable insights into the stability and reactivity of the molecule. (use the energy ordering of o2.) predict the bond order and make a sketch o. When the difference is really big, the bond becomes completely ionic, and the bonding mo basically is the lower energy. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Molecular shapes & valence bond theory mo theory: Web a molecular orbital interaction diagram shows how atomic or molecular orbitals combine together to. First, we need to determine the atomic orbitals of carbon (c) and oxygen (o). Its most important property is burning in air to give co 2, in the combustion of fossil fuels (use the energy ordering of o2. Predict the bond order and make a sketch of the lowest energy bonding molecul. (use the energy ordering of 02.) drag the. Relative ao energies in mo diagrams use ao energies to draw mo diagram to scale (more or less). Bonding in the co molecule. There are 3 steps to solve this one. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (figure 8.34). Not all targets will be filled. Web molecular orbital energy diagrams. Web refer to the mo diagram for co. Mo energy level diagram for h 2. The vertical axis represents energy and the thick bars represent orbitals. Mo theory • mo diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. Individual atomic orbitals (ao) are arranged on the far left and far right of the diagram. Web draw an mo energy diagram for co. Molecular orbital theory draw an mo energy diagram for co. Web energy heteronuclear diatomic molecules: Draw an mo energy diagram for co. (use the energy ordering of o $_{2.}$ A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. Its most important property is burning in air to give co 2, in the combustion of fossil fuels Web this problem has been solved! The homo of co is the 3σ mo. Web refer to the mo diagram for co. Not all targets will be filled.बi. (use the energy ordering of 02.) drag the. Ii 1 this problem has been solved! Draw a molecular orbital energy diagram for clf. Web this problem has been solved! Relative ao energies in mo diagrams use ao energies to draw mo diagram to scale (more or less). A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals. Carbon has the electron configuration 1s² 2s² 2p², and oxygen has the electron configuration 1s². Web a molecular orbital interaction diagram shows how atomic or molecular orbitals combine together to make new orbitals. Identify the homo and explain why co bonds to metal ions through the carbon atom rather than through the oxygen atom. Individual atomic orbitals (ao) are arranged on the far left and far right of the diagram. You can tell which orbitals are bonding because they have lower energy than the aos. (use the energy ordering of n2.) submitted by john s. Instant answer expert verified step 1/5 1. (use the energy ordering of 02.) drag the. Draw an mo energy diagram for co. Mo energy level diagram for h 2. Not all targets will be filled. The vertical axis represents energy and the thick bars represent orbitals. Labels can be used once, more than once, or not at all.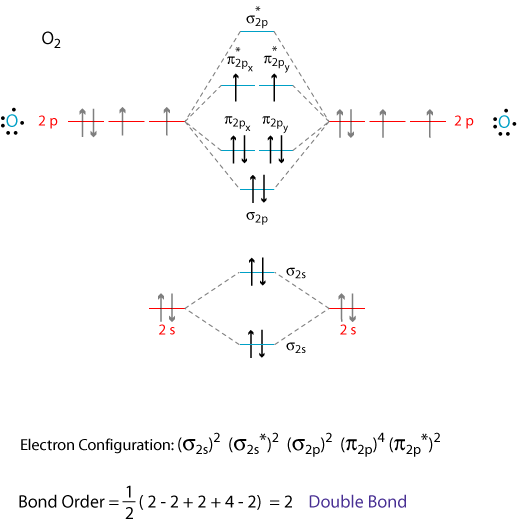
How do I draw the MO diagrams for "O"_2^ and "CO"^(+) and find out

draw an mo energy diagram for co howwideisafordtransitvan

draw an mo energy diagram for co shoppinggdiaper
MO Diagrams for Heterodiatomic Molecules Chemistry LibreTexts
Mo Diagram For Co
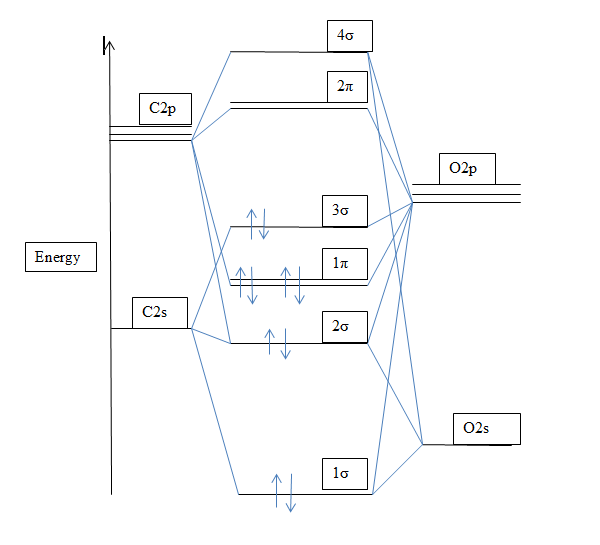
MO diagram of CO The Student Room
![[ANSWERED] Draw an MO energy diagram for CO Use the...](https://media.kunduz.com/media/sug-question-candidate/20220621013833830465-4634392.jpg?h=512)
[ANSWERED] Draw an MO energy diagram for CO Use the...
draw an mo energy diagram for co redandwhitevansmen
SOLVEDDraw an MO energy diagram for CO. (Use the energy ordering of O2

Aleks Drawing the MO energy diagram for a period 2 homodiatom YouTube
The Relative Energy Levels Of Atomic And Molecular Orbitals Are Typically Shown In A Molecular Orbital Diagram (Figure 8.34).
Ii 1 This Problem Has Been Solved!
(Assume That The Sp Orbitals Are Lower In Energy Than The P O.
Draw A Molecular Orbital Energy Diagram For Clf.
Related Post: