Draw Bohr Model Of An Atom
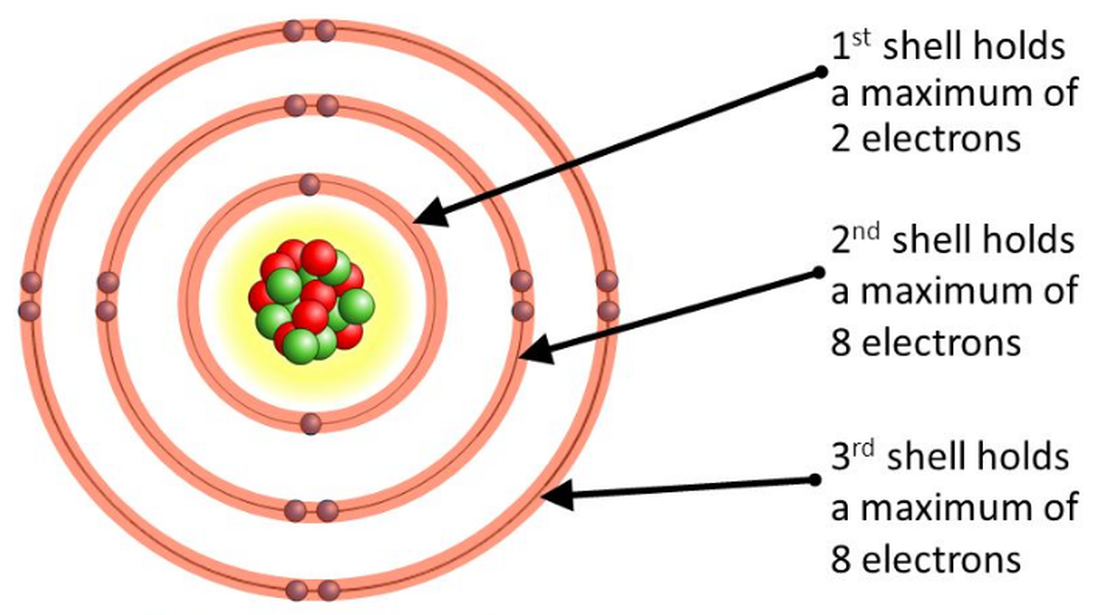
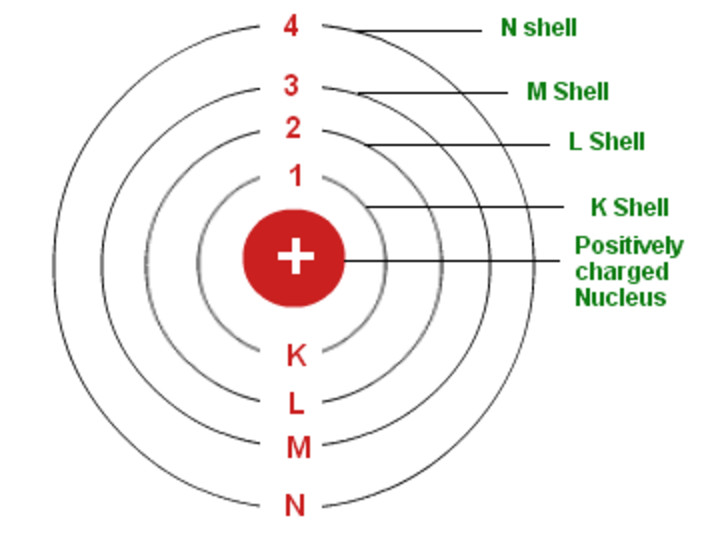
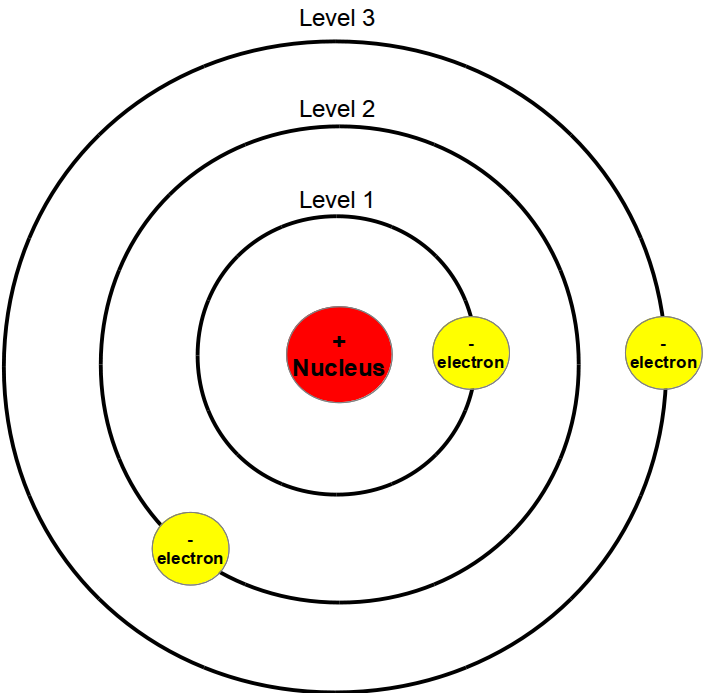
Draw Bohr Model Of An Atom - The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: Web niels bohr proposed a model of the atom and a model of the chemical bond. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons. Bohr's model of an atom. The maximum number of electrons that can fill each shell is: We’ll use a bohr diagram to visually represent where the electrons are around the nucleus.more. Web bohr’s model of the hydrogen atom structural model in which an electron moves around the nucleus only in circular orbits, each with a specific allowed radius; Orbitals in the bohr model. Web in this lesson i present an overview of: The bohr model of the atom was proposed by neil bohr in 1915. According to his model for a diatomic molecule, the electrons of the atoms of the molecule form a rotating ring whose plane is perpendicular to the axis of the. Information derived from the boron box: Unfortunately, bohr could not explain why the electron should be restricted to particular orbits. The following are his key contributions to our understanding of atomic. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. It came into existence with the modification of rutherford’s model of an atom. In order for an electron to be in the electron cloud of an atom, it must be in. Draw the nucleus of an atom. Discovery. The maximum number of electrons that can fill each shell is: Also, despite a great deal of tinkering, such as assuming that. Web in this video we'll look at the atomic structure and bohr model for the oxygen atom (o). Web bohr diagrams of atoms and ions electron shells. This packet includes a video explaining the bohr model and how. The boron family is located in the 13 th group of the periodic table: Web in this lesson i present an overview of: Web bohr’s model of the hydrogen atom structural model in which an electron moves around the nucleus only in circular orbits, each with a specific allowed radius; In order for an electron to be in the electron. According to his model for a diatomic molecule, the electrons of the atoms of the molecule form a rotating ring whose plane is perpendicular to the axis of the. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. According to the bohr atomic model, a small positively. Draw the second electron shell,. Web what is bohr’s model of an atom? Web to review the bohr model of an atom. Web bohr’s model of the hydrogen atom structural model in which an electron moves around the nucleus only in circular orbits, each with a specific allowed radius; The orbiting electron does not normally emit electromagnetic radiation, but does. Orbitals in the bohr model. Web steps to draw bohr model of boron. Overview of the bohr model niels bohr proposed the bohr model of the atom in 1915. Web the bohr model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. The steps to drawing bohr diagrams for elements and atoms are. Web bohr's key idea in his model of the atom is that electrons occupy definite orbitals that require the electron to have a specific amount of energy. Web what is bohr’s model of an atom? The maximum number of electrons that can fill each shell is: Draw the second electron shell,. The protons and neutrons are placed into the nucleus. E ( n) = − 1 n 2 ⋅ 13.6 ev He concluded that electron will have more energy if it is located away from the nucleus whereas electrons will have less energy if it located near the nucleus. Draw the first electron shell and put the electrons as a dot in it. Class 9 chemistry (india) >. Also, despite. The steps to drawing bohr diagrams for elements and atoms are. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. Web. The orbiting electron does not normally emit electromagnetic radiation, but does so when changing from one orbit to another. He concluded that electron will have more energy if it is located away from the nucleus whereas electrons will have less energy if it located near the nucleus. Web what is bohr’s model of an atom? One of the allowable orbitals and it must have the precise energy required for that orbit. Web how to draw bohr model of an atom? Electrons must occupy the lowest available shell, closest to the nucleus. The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: We’ll use a bohr diagram to visually represent where the electrons are around the nucleus.more. Web in this video we'll look at the atomic structure and bohr model for the oxygen atom (o). Web what is bohr’s model of an atom? Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons. The boron family is located in the 13 th group of the periodic table: According to his model for a diatomic molecule, the electrons of the atoms of the molecule form a rotating ring whose plane is perpendicular to the axis of the. Information derived from the boron box: In order for an electron to be in the electron cloud of an atom, it must be in. Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum.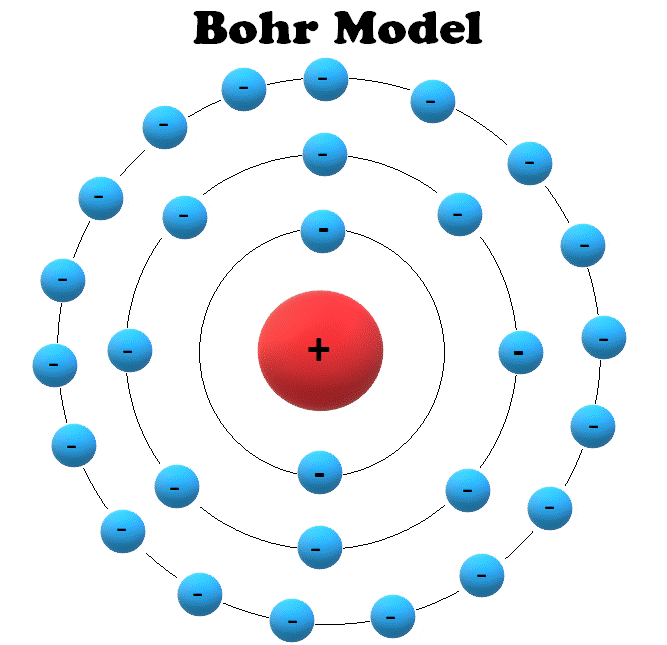
Atoms and Electrons Electronics Reference

Bohr Model of the Hydrogen Atom Postulates, Limitations Embibe
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom
Unreal Truths Matter Waves and the Bohr Model of the Atom
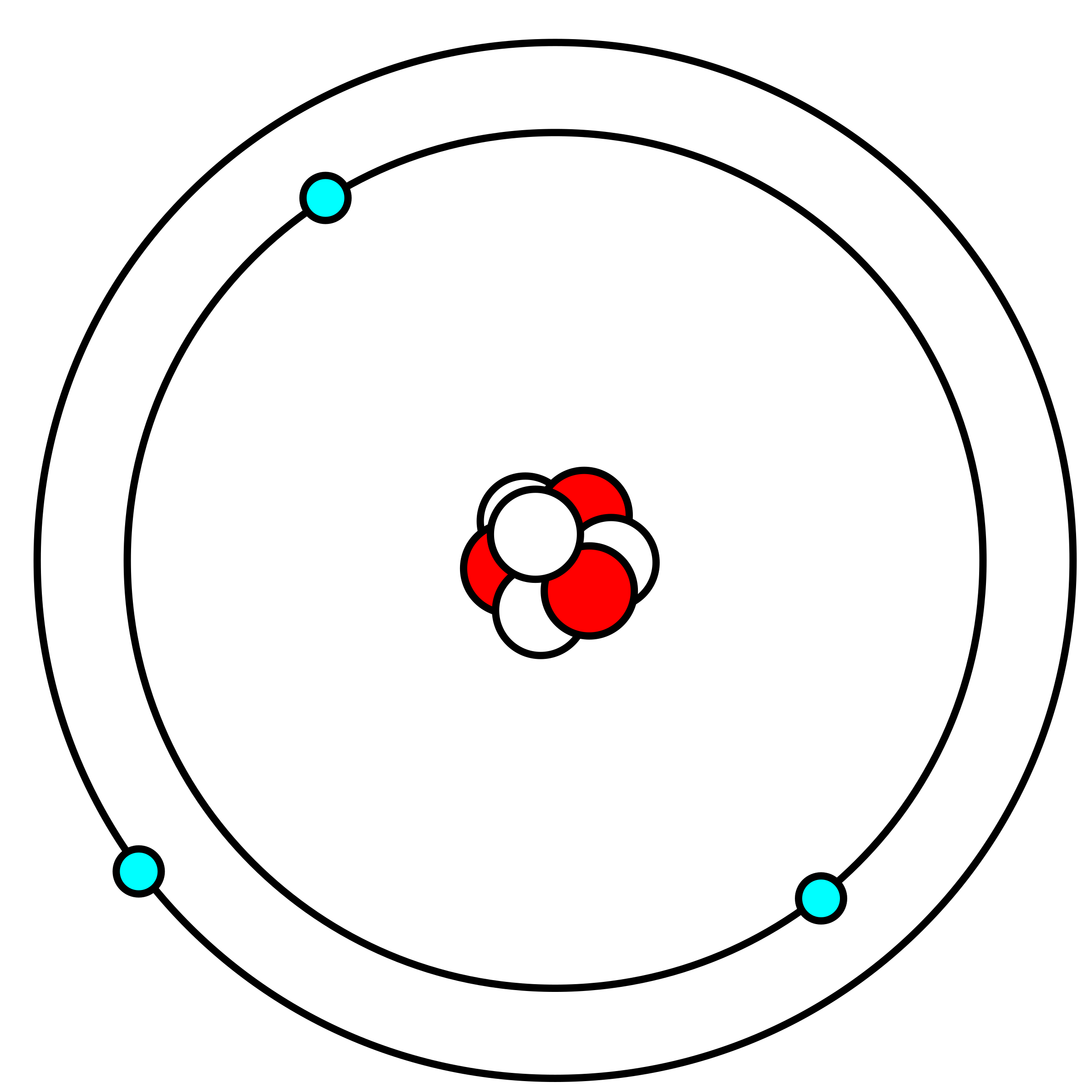
Clipart Lithium atom in Bohr model
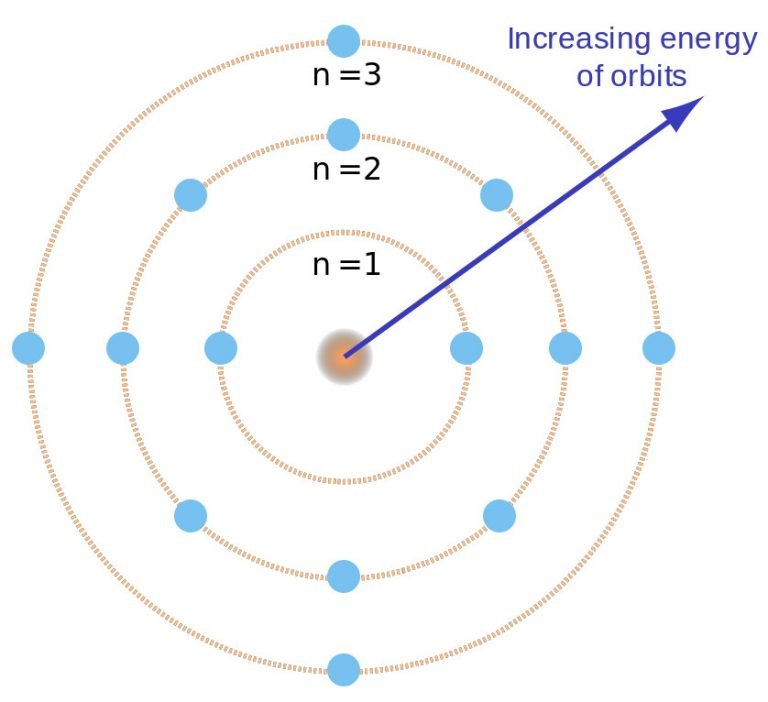
Niel Bohr's Atomic Theory Explained

Describe Bohr’s model of the hydrogen atom. bitWise Academy

draw a bohr model of an oxygen atom artvansaletoday
Bohr Model of the Atom Overview and Examples
Web In The Bohr Model, There Are A Few Rules That Will Help You Draw Accurate Diagrams.
The Protons And Neutrons Are Placed Into The Nucleus And The.
The Maximum Number Of Electrons That Can Fill Each Shell Is:
We’ll Use A Bohr Diagram To Visually Represent Where The Electrons Are Around The Nucleus Of The Oatom.
Related Post: