Draw The Electron Configuration For A Neutral Atom Of Argon.
Draw The Electron Configuration For A Neutral Atom Of Argon. - 1s 2 2s 2 2p 6 3s 1: 1s 2 2s 2 2p 5: Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the. Write the electron configuration for a neutral atom of argon | 0. Draw the electron configuration for a neutral atom of argon. Web our videos prepare you to succeed in your college classes. Web the arrangement of electrons in argon in specific rules in different orbits and orbitals is called the electron configuration of argon. Find the element on the periodic table. In aufbau principle, the electrons are filled according to the increasing energy level of orbitals. Web draw the electron configuration for a neutral atom of argon. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Electron configuration of neon (ne) [he] 2s 2 2p 6: Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? In aufbau principle, the electrons are filled according to the increasing energy level of orbitals. Web faq. Web the four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Our videos will help you understand concepts, solve. The electron configurations and orbital diagrams of these four elements are: We can represent this as 1s². Web the electron configuration and orbital diagram for carbon are: Web the neutral atom chlorine (z=17), for instance has 17 electrons. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of argon. This question hasn't been solved yet! Remember that potassium is element number 19 so has 19 electrons. Draw a small circle and write the symbol in the centre. The next six electrons will go in the 2p orbital. Write the electron configuration for an argon cation with a charge of +2. This problem has been solved! 1s 2 2s 2 2p 6: Draw the electronic configuration for potassium using the electron configuration diagram below. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web so, in neutral atom of argon have 18 electron. Find the element on the periodic table. Draw the electron configuration for a neutral atom of argon. Web an electrically neutral atom has the following electron configuration: Web draw the electron configuration for a neutral atom of argon. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. The electron configurations and orbital diagrams of these four elements are: Our videos will help you understand concepts, solve your homework, and do great on your exams. As stated, the electron configuration of each element is unique to its position on the periodic table. You'll get a detailed solution from a subject matter expert. [ne] 35 3p² what is the chemical symbol for the atom? Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? 1s 2 2s 2 2p 4: Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Let us help you simplify your studying. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of argon. Web electron affinity the energy released when an electron is added to the neutral. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of argon. Web the arrangement of electrons in argon in specific rules in different orbits and orbitals is called the electron configuration of argon. In writing the electron configuration for argon the first two electrons will go in the 1s orbital. Write the electron configuration. This problem has been solved! If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! 1s 2 2s 2 2p 5: Since 1s can only hold two electrons the next 2 electrons for argon go in the 2s orbital. Write the electron configuration for a neutral atom of argon | 0. Web our videos prepare you to succeed in your college classes. The electron configuration of argon is [ ne] 3s 2 3p 6 , if the electron arrangement is through orbitals. Remember that potassium is element number 19 so has 19 electrons. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Let us help you simplify your studying. Draw the electron configuration for a neutral atom of argon. A neutral chlorine atom has 17 electrons. Web an electrically neutral atom has the following electron configuration: Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of argon. Web the electron configuration and orbital diagram for carbon are: 1s 2 2s 2 2p 6 3s 1:/argonatom-58b44b6f5f9b586046e5840d.jpg)
Atoms Diagrams Electron Configurations of Elements
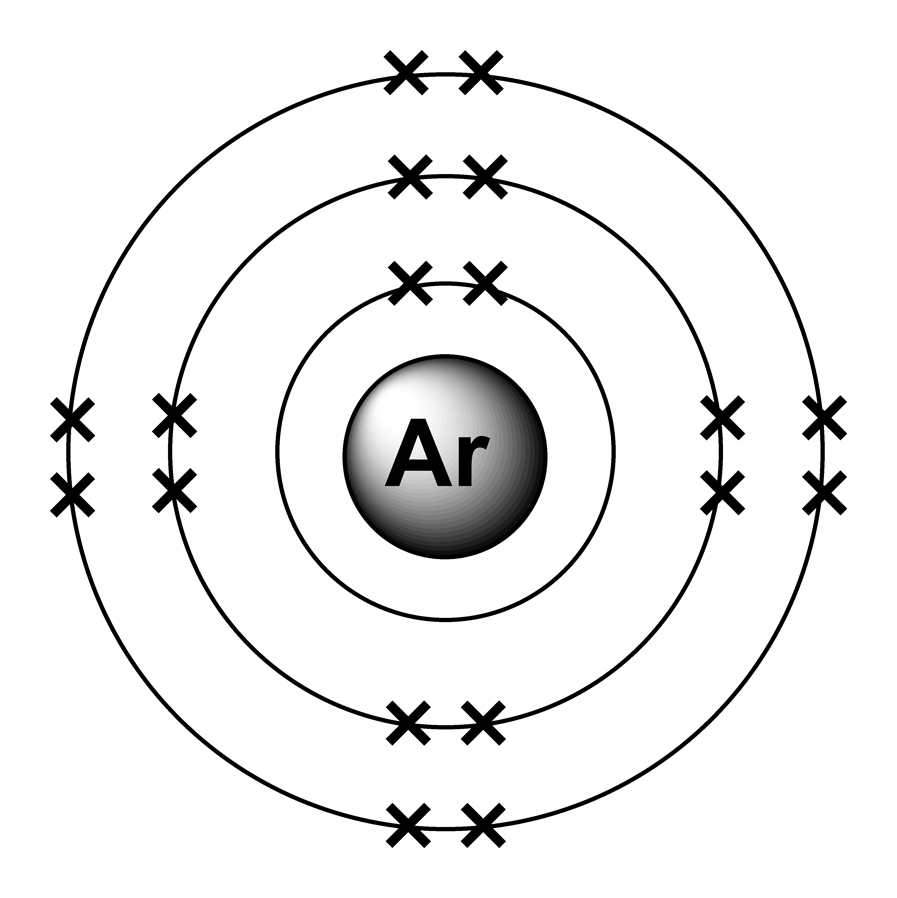
Argon Argon Valence Electron Configuration
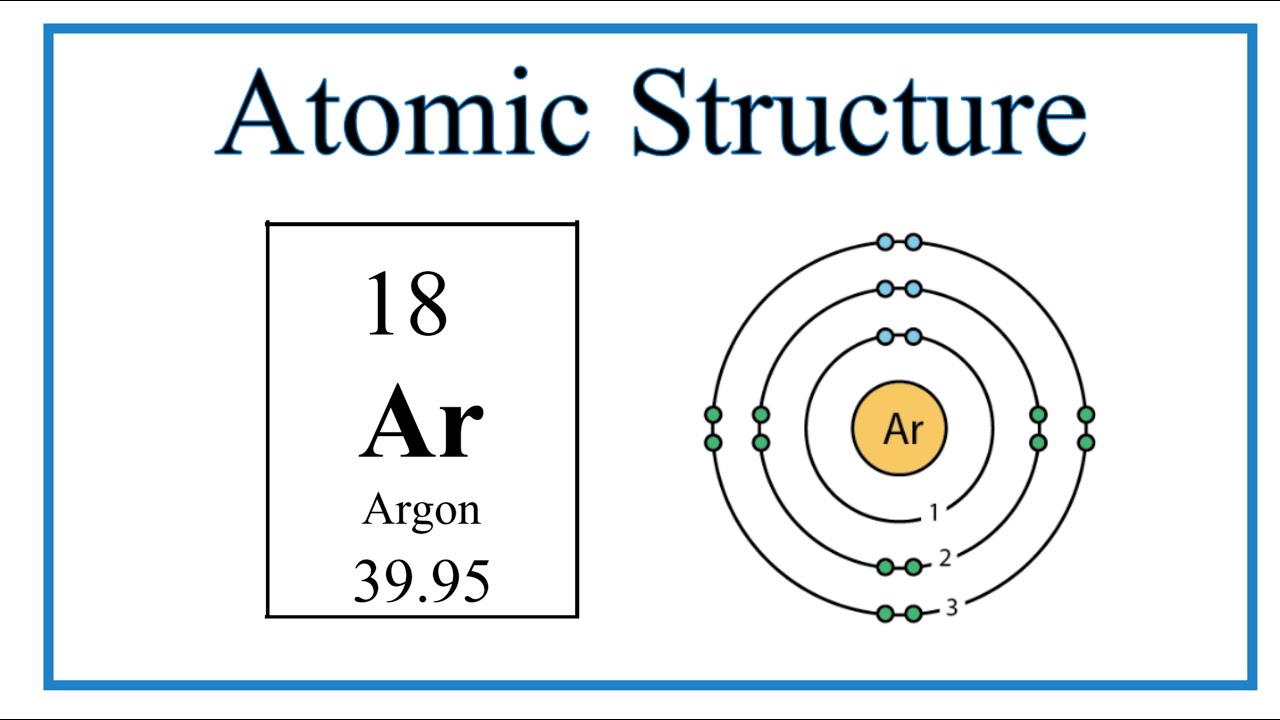
Atomic Structure (Bohr Model) for Argon (Ar) YouTube
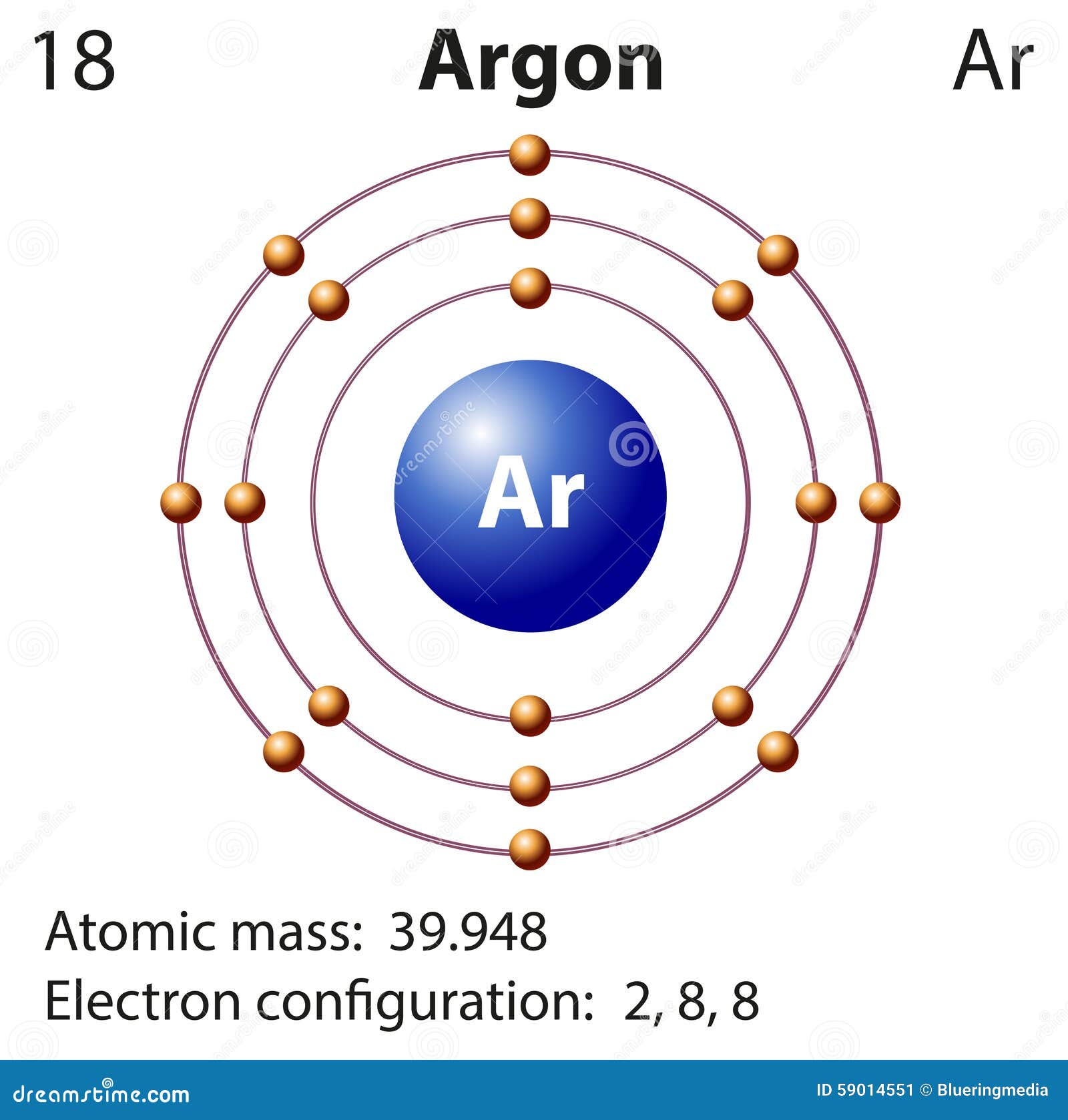
Argon Argon Electron Configuration

Argon, atomic structure Stock Image C018/3699 Science Photo Library
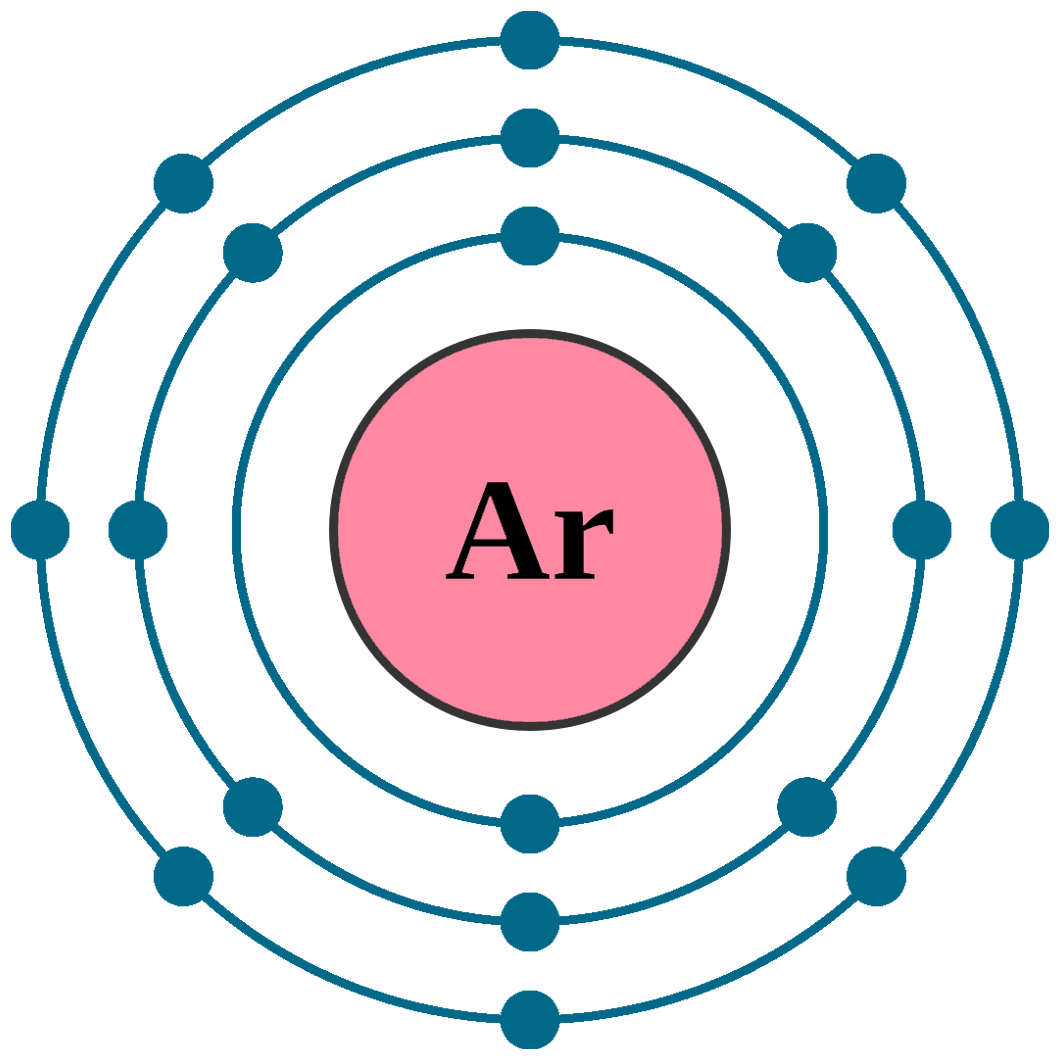
Argon electron configuration Newton Desk
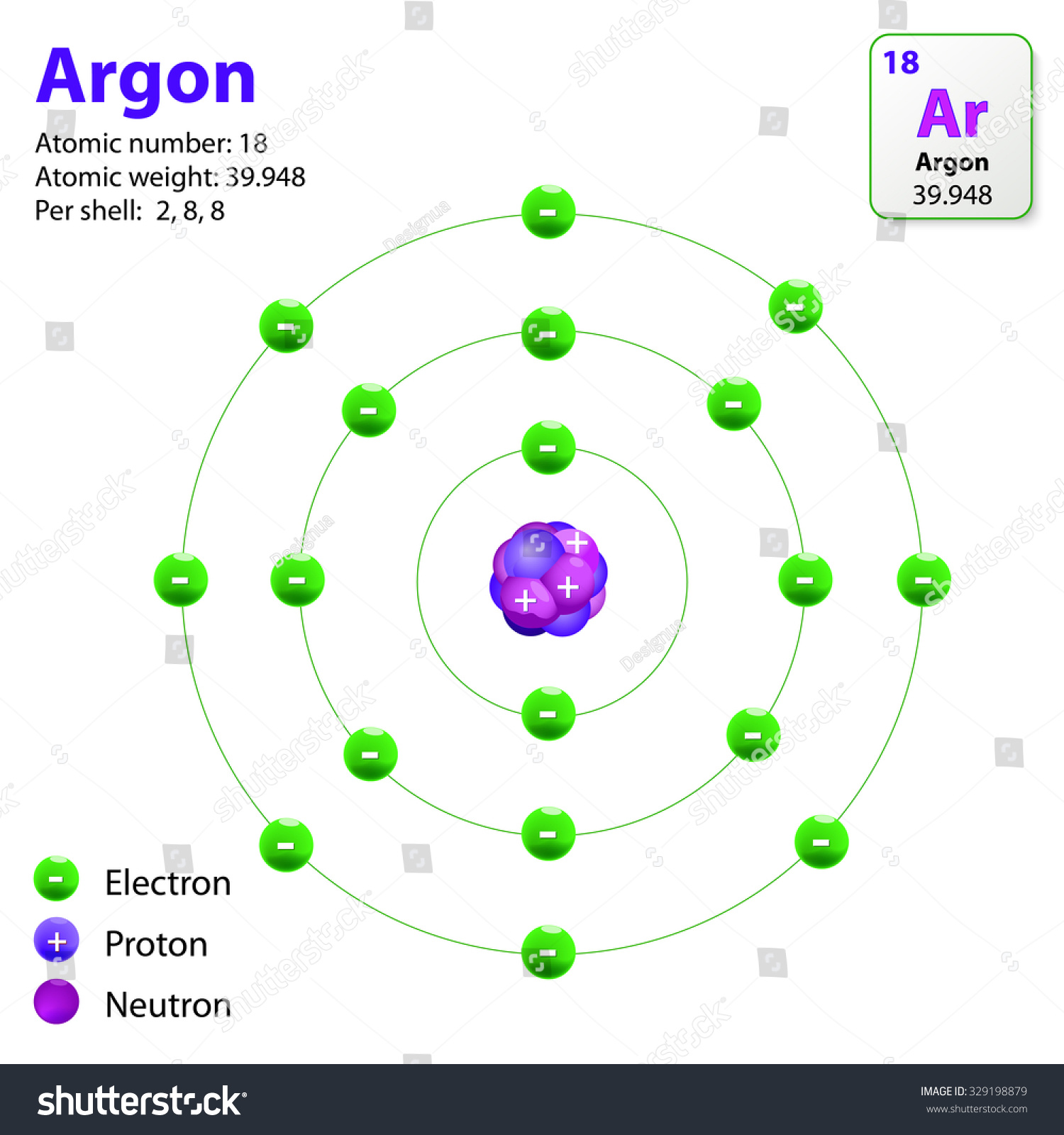
This Diagram Shows Electron Shell Configuration Stock Vector 329198879

Draw The Electron Configuration For A Neutral Atom, HD Png Download
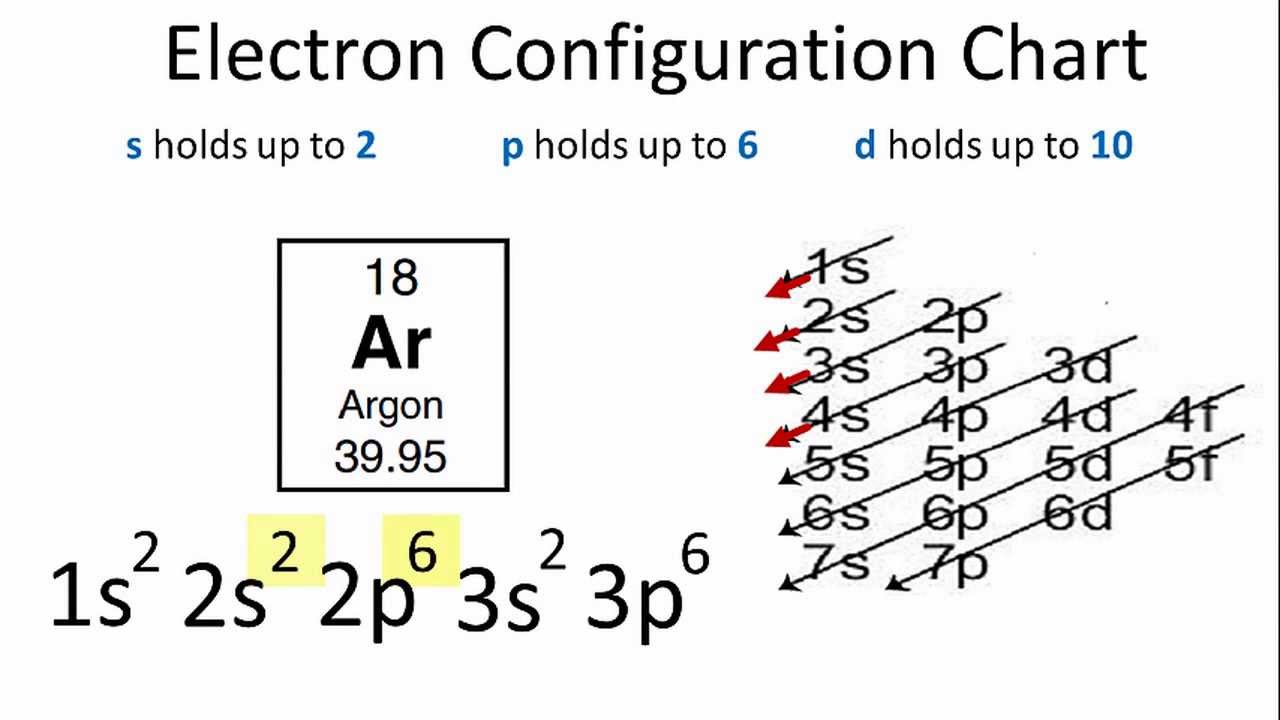
Argon Electron Configuration YouTube

Argon Protons Neutrons Electrons Electron Configuration
Web What Is The Electron Configuration Of A Neutral Chlorine Atom?
We Can Represent This As 1S².
In Aufbau Principle, The Electrons Are Filled According To The Increasing Energy Level Of Orbitals.
The Alkali Metal Sodium (Atomic Number 11) Has One More Electron Than The Neon Atom.
Related Post: