Draw The Electron Configuration For A Neutral Atom Of Calcium.
Draw The Electron Configuration For A Neutral Atom Of Calcium. - Therefore, the number of electrons in neutral atom of calcium is 20. Web the upper right side shows the number of electrons in a neutral atom. Web an electrically neutral atom has the following electron configuration: Energy х this problem has been solved! Web this means that the electron configuration for calcium must end with 4s2. Web and thus for the neutral atom, we have 20 electrons to distribute: What is the name of this atom? Simply use this information to obtain its electronic configuration. Web electrons and electron configuration. How do you draw the shell diagram of sodium atom? There is one unpaired electron. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1s 2 2s 2 2p 1: Remember, a neutral atom contains the same number of protons and electrons. Web electron configuration of beryllium (be) [he] 2s 2: Lliruns in aiums and molecules drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of calcium. Web the electron configuration of calcium is [ ar] 4s 2 , if the electron arrangement is through orbitals. Typically, you need at least 8 steps to determine the electron configuration, starting with finding. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2) how do we write the configuration for ca^ (2+)? In the case of calcium this is 4s2. Web this means that the electron configuration for calcium must end with 4s2. Hence, calcium has 2 valence electrons. Electron configuration of boron (b) [he] 2s 2 2p 1: Put the noble gas in brackets and write the remainder of the electron configuration. Web calcium has an atomic number of 20. Locate the noble gas element in the period above the element of interest. Web what is the electron configuration of a neutral atom of sodium (na)? Therefore, the number of electrons in neutral atom of calcium is 20. Hence, calcium has 2 valence electrons. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Web calcium has an atomic number of 20. What about potassium, and what about for chlorine? A neutral chlorine atom has 17 electrons. Web the electron configuration and the orbital diagram are: 1s 2 2s 2 2p 1: 1s 2 2s 2 2p 2: Web electrons and electron configuration. Web the electron configuration of calcium is [ ar] 4s 2 , if the electron arrangement is through orbitals. Web an electrically neutral atom has the following electron configuration: 1s 2 2s 2 2p 3: Put the noble gas in brackets and write the remainder of the electron configuration. The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). Continue the electron configuration from the noble gas until. Therefore, the number of electrons in neutral atom of calcium is 20. Web chemistry questions and answers. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. The next six electrons will go in the 2p orbital. 1s^2 2s^2 2p^6 3s^2. The electron configuration follows the order: The atomic number of cl is 17. Web draw the electron configuration for a neutral atom of calcium. Locate the noble gas element in the period above the element of interest. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. This means the first shell (1s) has 2. Locate the noble gas element in the period above the element of interest. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. How many core electrons are there? Web draw the electron configuration for a neutral calcium atom. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Web the electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. Continue the electron configuration from the noble gas until you reach the element of interest. An atom has a valence shell electron configuration of #ns^1#. How do you draw the shell diagram of sodium atom? Web and thus for the neutral atom, we have 20 electrons to distribute: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s,. Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2. What about potassium, and what about for chlorine? Web draw the electron configuration for a neutral calcium atom. Electron configuration of carbon (c) [he] 2s 2 2p 2: The helium atom contains two protons and two electrons. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). In the case of calcium this is 4s2.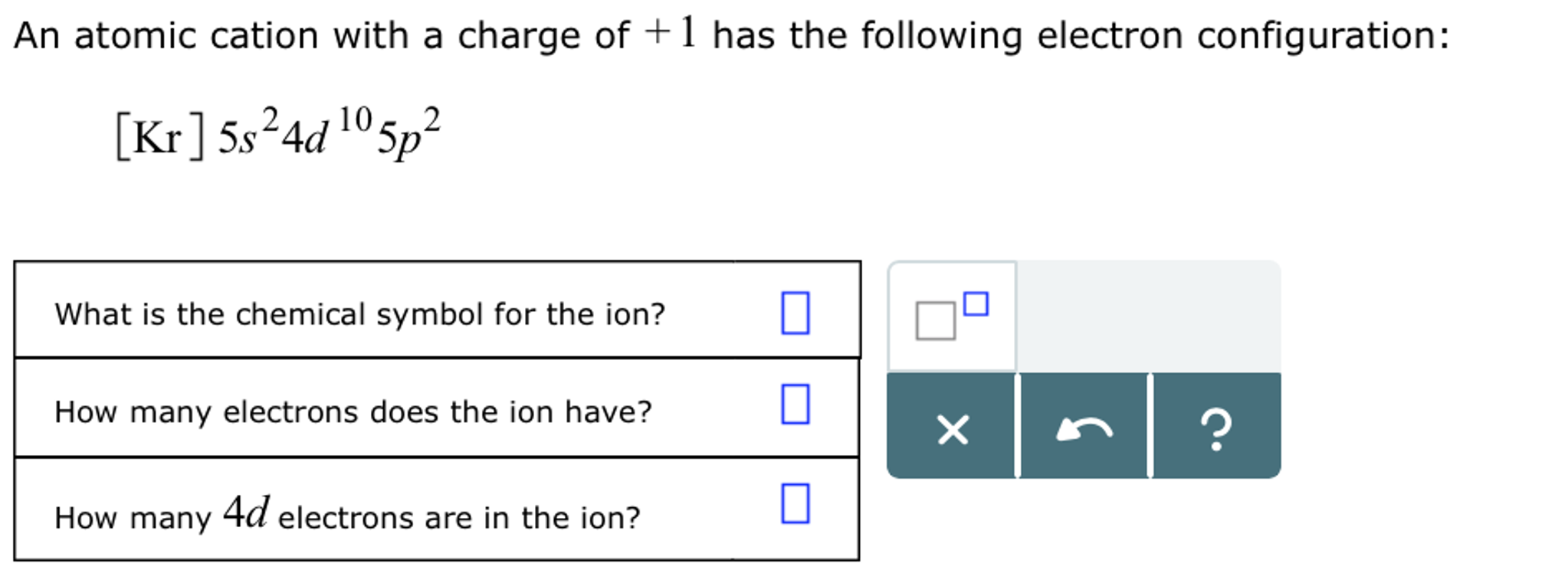
(Get Answer) Draw The Electron Configuration For A Neutral Atom Of

Calcium (Ca) electron configuration and orbital diagram (2023)

Bohr Diagram For Calcium
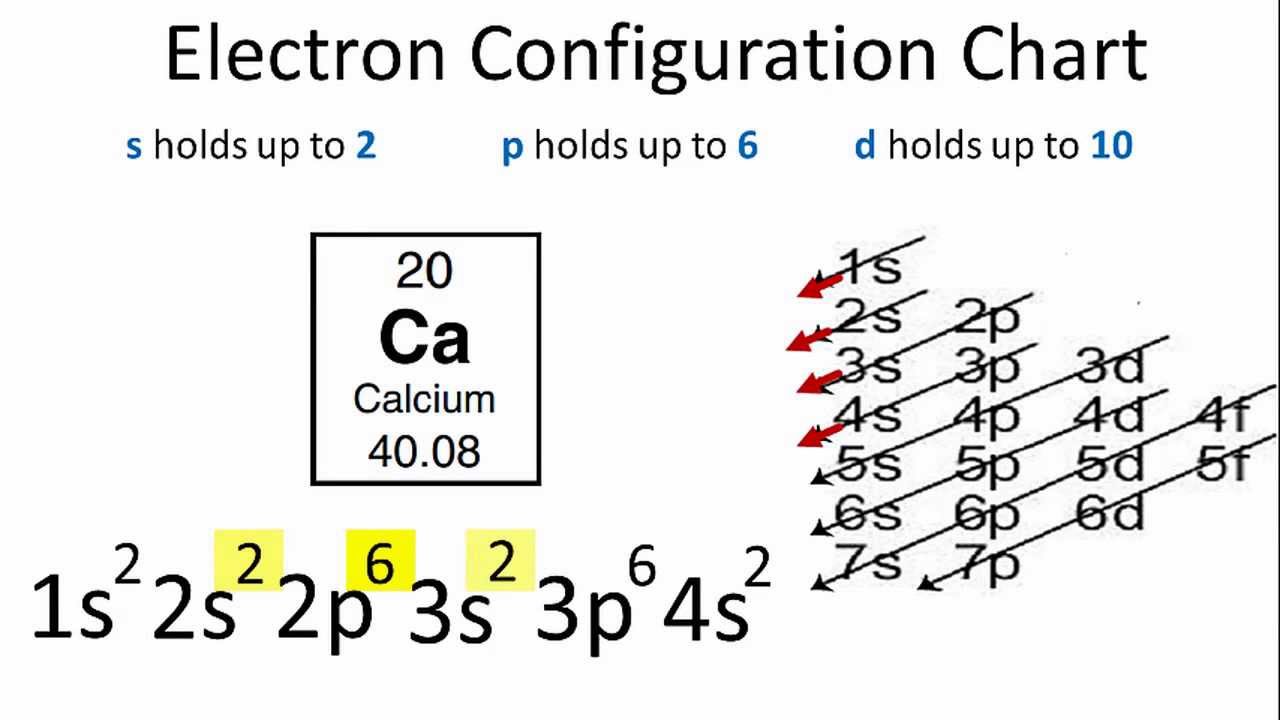
Calcium Electron Configuration (Ca) with Orbital Diagram
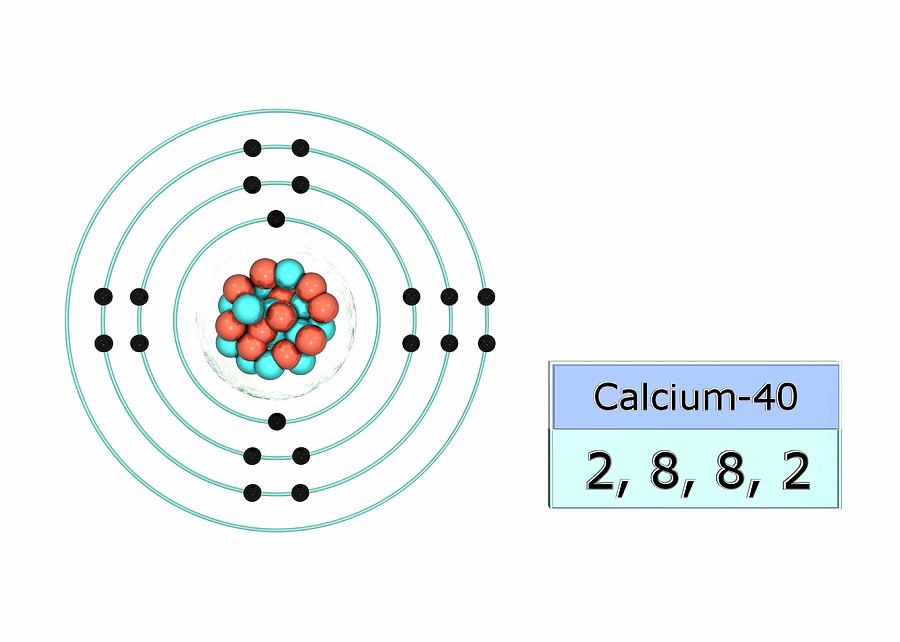
Calcium Electron Dot Diagram Photos Cantik

How Many Valence Electrons are in Calcium Periodic Table
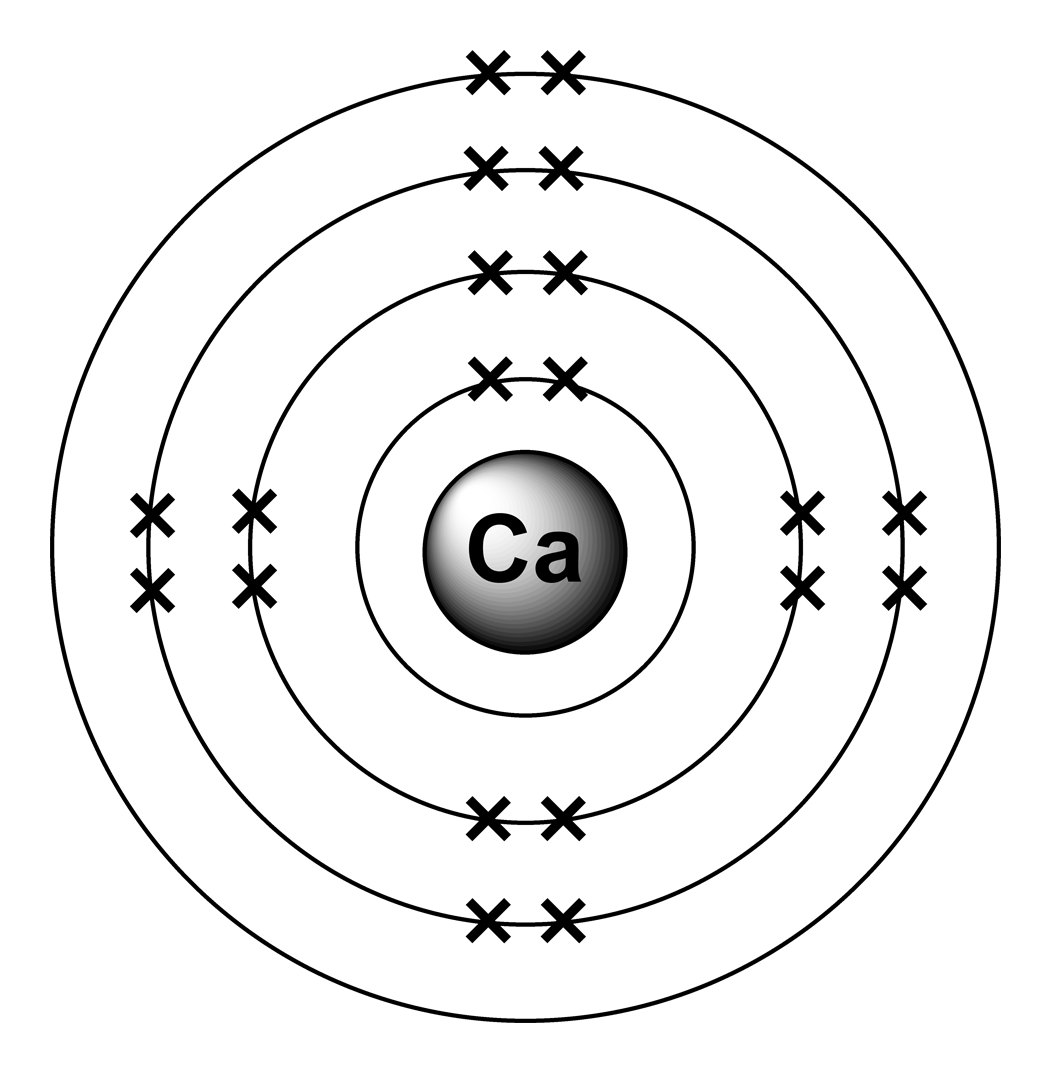
Electron arrangements

Calcium, atomic structure Stock Image C018/3701 Science Photo Library
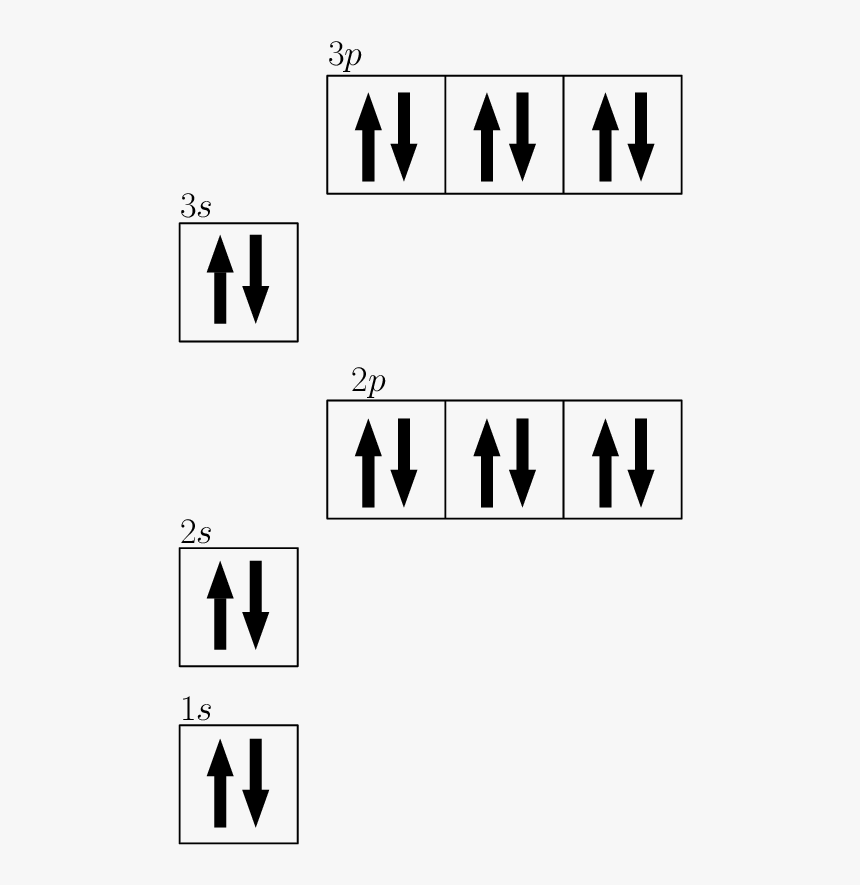
Draw The Electron Configuration For A Neutral Atom, HD Png Download

Calcium Facts
The Arrangement Of An Element’s Electrons Tells You Where It Is On The Periodic Table.
1S 2 2S 2 2P 1:
Web In Writing The Electron Configuration For Calcium The First Two Electrons Will Go In The 1S Orbital.
Web Electrons And Electron Configuration.
Related Post: