Draw The Electron Configuration For A Neutral Atom Of Scandium
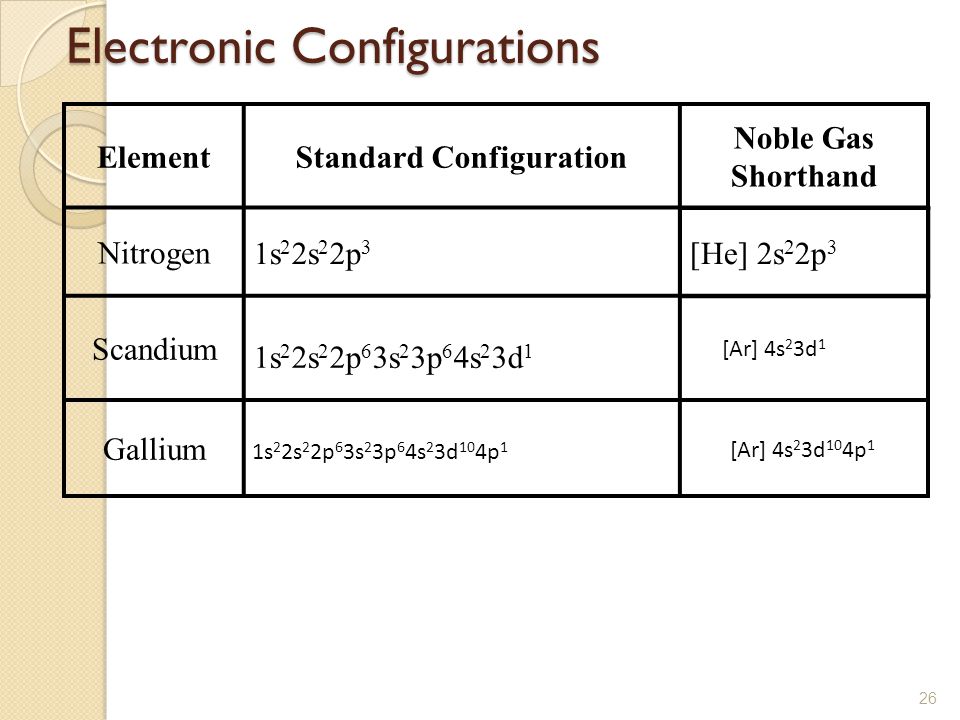
Draw The Electron Configuration For A Neutral Atom Of Scandium - The electron configuration of a neutral atom of scandium is 1s2 2s2 2p6 3s2 3p6 4s2 3d1. This turns out to be argon 4s 1, 3d 1 or once again you could write argon, 3d 1, 4s 1. You will get the detailed information about the periodic table which will convert a newbie into pro. Scandium metal commonly forms sc^ (3+) ions. You will also get the hd images of the periodic table (for free). 3d^ (1)4s^2) but this really doesn't matter so much. Web so, the scandium atom is neutral, hence, its number of electrons will be equal to the number of protons which is 21 as we already discussed. Thus, it is simple to determine the charge on such a negative ion: It has 21 protons and if it is neutral, it's also gonna have one more electron relative to a neutral calcium atom. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. You can effortlessly find every single detail about the elements from this single interactive periodic table. Instead of having three electrons in the outer shell, scandium adds its electron to the second to last shell. Energy 3p click to change la. Thus, it. Web the electron configuration and the orbital diagram are: We're asked to find the electron configuration for scandium. You can effortlessly find every single detail about the elements from this single interactive periodic table. Draw the electron configuration for a neutral atom of scandium video answer: Scandium metal commonly forms sc^ (3+) ions. Web electrons and electron configuration. Web the electron configuration of scandium is [ ar] 3d 1 4s 2 , if the electron arrangement is through orbitals. Energy 3p click to change la. Web now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher. Following hydrogen. The valence orbitals may have different ordering (i.e. The atomic number of titanium is 22 and atomic number os scandiu. Web now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher. The first electron has the same four quantum numbers as the hydrogen atom electron. Scandium electron configuration using the aufbau principle a scandium atom is a neutral atom that has an atomic number of 21 which implies it has a total of 21 electrons. The ground state abbreviated electronic configuration of neutral. The electron configuration of a neutral atom of scandium (sc) can be determined by using the periodic table. Web learn about the. Web every element adds one more electron to the outermost shell. Web electrons and electron configuration. So some things that you need to note along the left side, these rows are numbered and those numbers match up with what is over here. Web learn about the electron configuration diagram for scandium, a transition metal commonly found in minerals and used. Understand the arrangement of electrons in scandium's energy levels and orbital shells, providing insights into its chemical reactivity and bonding behavior. Web electrons and electron configuration. Thus, it is simple to determine the charge on such a negative ion: Instead of having three electrons in the outer shell, scandium adds its electron to the second to last shell. You can. The helium atom contains two protons and two electrons. 83% (24 ratings) transcribed image text: Web for example if you form the scandium plus one ion, the electron configuration for the scandium plus one ion, so we're losing an electron from a neutral scandium atom. You will get the detailed information about the periodic table which will convert a newbie. You will also get the hd images of the periodic table (for free). Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Draw the electron configuration for a neutral atom of boron. Therefore, the number of electrons. Draw the electron configuration for a neutral atom of scandium. Alternatively, it can be represented as [ne] 3s2 3p6 4s2 3d1. The valence orbitals may have different ordering (i.e. 3d^ (1)4s^2) but this really doesn't matter so much. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. 3d^ (1)4s^2) but this really doesn't matter so much. And so, it could have a similar electron configuration. You will find scandium to the right of calcium in the fourth period of the table. O electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of scandium. Web electrons and electron configuration. (b) how many valence electrons are found in this element? Make sure to label each sublevel and populate with the appropriate numbers of electrons. Web for example if you form the scandium plus one ion, the electron configuration for the scandium plus one ion, so we're losing an electron from a neutral scandium atom. Scandium electron configuration using the aufbau principle a scandium atom is a neutral atom that has an atomic number of 21 which implies it has a total of 21 electrons. Following hydrogen is the noble gas helium, which has an atomic number of 2. Energy 3p click to change la. Web answer link sc, z=21 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2)3d^1 we account for 21 electrons. The electron configuration of a neutral atom of scandium (sc) can be determined by using the periodic table. Web now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher. Pause this video and think about that.
Orbital Diagram For Scandium
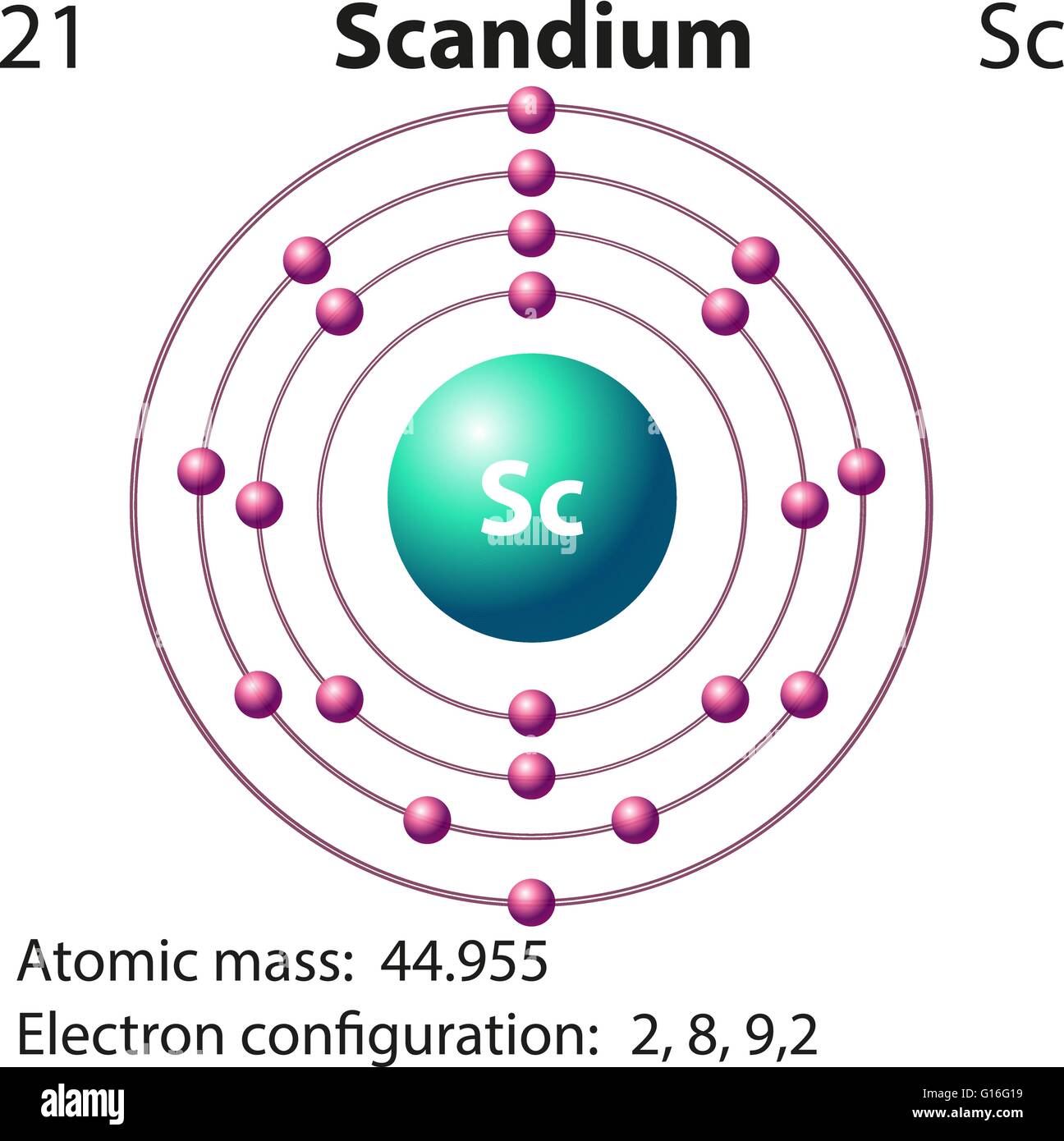
Symbol and electron diagram for Scandium illustration Stock Vector Art

Scandium Atom Science Notes and Projects
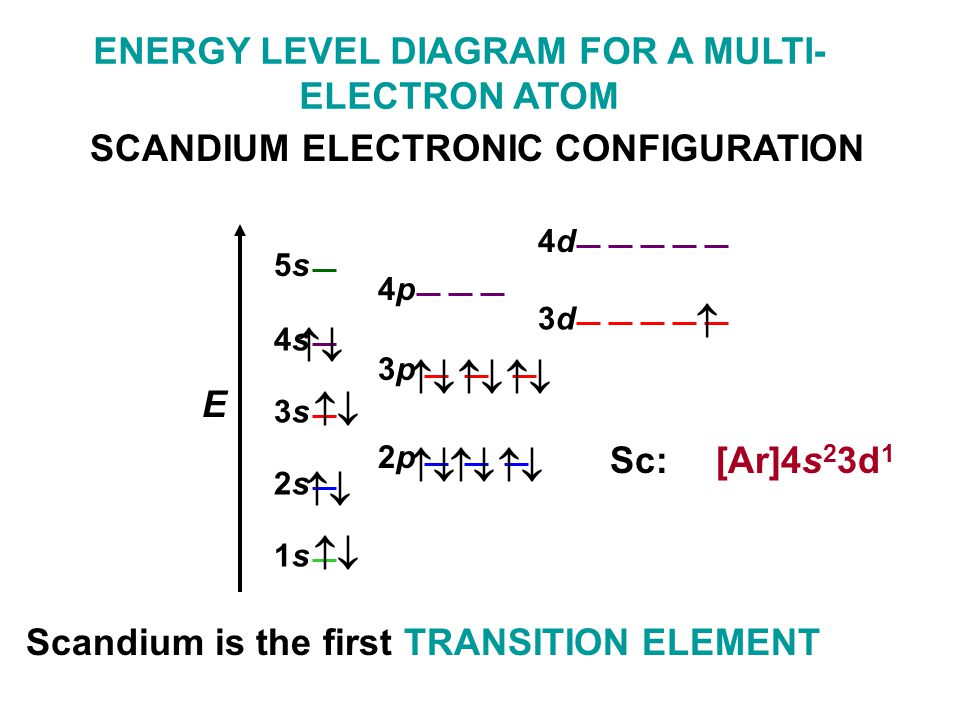
Scandium Electron Configuration (Sc) with Orbital Diagram
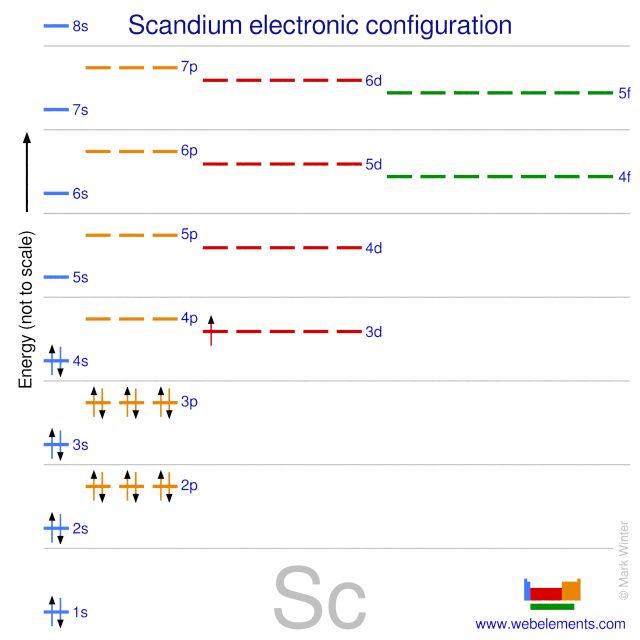
WebElements Periodic Table » Scandium » properties of free atoms

Pin on Chemistry

Scandium electronic configuration How to Write Scandium electronic
Scandium electron configuration Stock Image C029/5028 Science Photo
:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)
Atoms Diagrams Electron Configurations of Elements
Scandium Electron Configuration (Sc) with Orbital Diagram
Web So, The Scandium Atom Is Neutral, Hence, Its Number Of Electrons Will Be Equal To The Number Of Protons Which Is 21 As We Already Discussed.
Understand The Arrangement Of Electrons In Scandium's Energy Levels And Orbital Shells, Providing Insights Into Its Chemical Reactivity And Bonding Behavior.
Web The Electron Configuration Of Scandium Is [ Ar] 3D 1 4S 2 , If The Electron Arrangement Is Through Orbitals.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post:
