Draw The Electron Dot Formula For Sicl2Br2
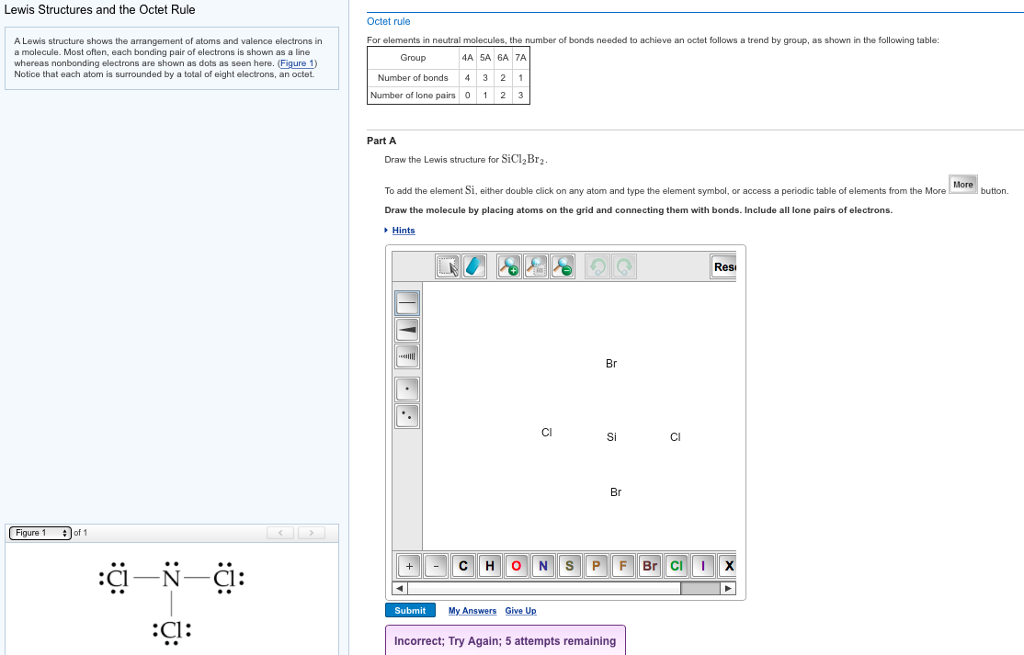
Draw The Electron Dot Formula For Sicl2Br2 - So, if you are ready to go with these 6 simple steps, then let’s dive right into it! In the typical lecture, you end up with the final formula but you may not remember all of the individual steps that were done in drawing the formula. The total number of valence electrons is calculated and used to form single bonds between si and each of the cl and br atoms. Include all lone pairs of electrons. Web how do you draw a lewis structure for sicl_2br_2? These tutorials allow you to repeat any step of the process. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability how to draw lewis structure of sicl 2 br 2? Draw the molecule by placing atoms on the grid and connecting them with bonds. To add the element si, double click on any atom while the bond tab, is highlighted, type the element symbol, and hit enter. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Valance electron of chlorine is 7 (3s 2 3p 5) and bromine also 7 (4s 2 4p 5 ). To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more more button button. To add the element si, double click on any atom while the. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the. This problem has been solved! Include all lone pairs of electrons. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Web how do you draw a lewis structure for sicl_2br_2? Include all lone pairs of electrons. Firstly, silicon (si) is identified as the least electronegative and the central atom in the sicl2br2 molecule. This is done by adding the valence shell electrons of all the constituent atoms. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that. Draw the molecule by placing atoms on the grid and connecting them with bonds. Firstly, silicon (si) is identified as the least electronegative and the central atom in the sicl2br2 molecule. Silicon has total 4 electrons in its outer most shell (3s 2 3p 2 ). Include all lone pairs of electrons. You'll get a detailed solution from a subject. Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has. These tutorials allow you to repeat any step of the process. Web contents how to draw lewis structure of sicl2br2? Steps to draw the lewis structure of sicl2br2. Draw the molecule by placing atoms on the grid and connecting them with bonds. How do you draw a lewis structure for sicl_2br_2? Web it is calculated using the formula given below. Include all lone pairs of electrons. Web in the sicl 2 br 2 lewis structure, there are four single bonds around the silicon atom, with two chlorine atoms and two bromine atoms attached to it, and on each chlorine and bromine atom,. Web in the sicl 2 br 2 lewis structure, there are four single bonds around the silicon atom, with two chlorine atoms and two bromine atoms attached to it, and on each chlorine and bromine atom, there are three lone pairs. Include all lone pairs of electrons this problem has been solved! Web draw the lewis structure for sicl2br2. Draw. Expert answer 100% (72 ratings) Silicon has total 4 electrons in its outer most shell (3s 2 3p 2 ). Find the total valence electrons in sicl2br2 molecule in order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom.. Web part a draw the lewis structure for sicl2br2. Draw the molecule by placing atoms on the grid and connecting them with bonds. Valance electron of chlorine is 7 (3s 2 3p 5) and bromine also 7 (4s 2 4p 5 ). 1 become a study.com member to unlock this answer! Silicon (si) has 4 valence electrons, chlorine (cl) has. To add the element si, double click on any atom while the bond tab, is highlighted, type the element symbol, and hit enter. Web in the sicl 2 br 2 lewis structure, there are four single bonds around the silicon atom, with two chlorine atoms and two bromine atoms attached to it, and on each chlorine and bromine atom, there are three lone pairs. Firstly, silicon (si) is identified as the least electronegative and the central atom in the sicl2br2 molecule. In the typical lecture, you end up with the final formula but you may not remember all of the individual steps that were done in drawing the formula. Determine the total number of valence electrons in the molecule. This is done by adding the valence shell electrons of all the constituent atoms. How to draw the lewis structure for sicl2br2 watch on contents steps Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has. Draw the molecule by placing atoms on the grid and connecting them with bonds. Here, i have explained 6 simple steps to draw the lewis dot structure of sicl2br2 (along with images). To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more more button button. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Draw the molecule by placing atoms on. Include all lone pairs of electrons. The remaining electrons are distributed as lone pairs around the chlorine and bromine atoms.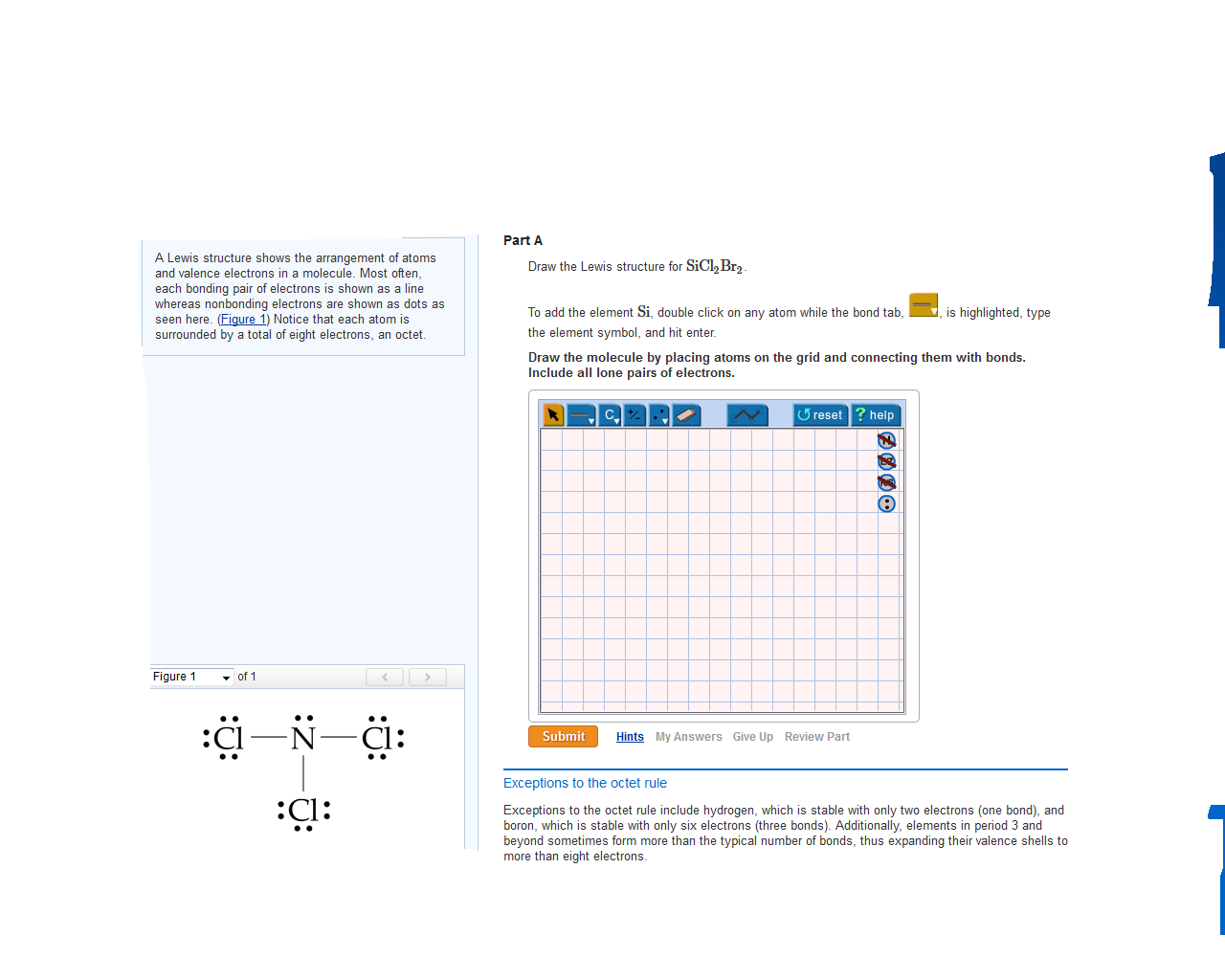
Sicl2br2 Lewis Structure How To Draw The Lewis Structure
Draw The Lewis Structure For Sicl2br2

SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
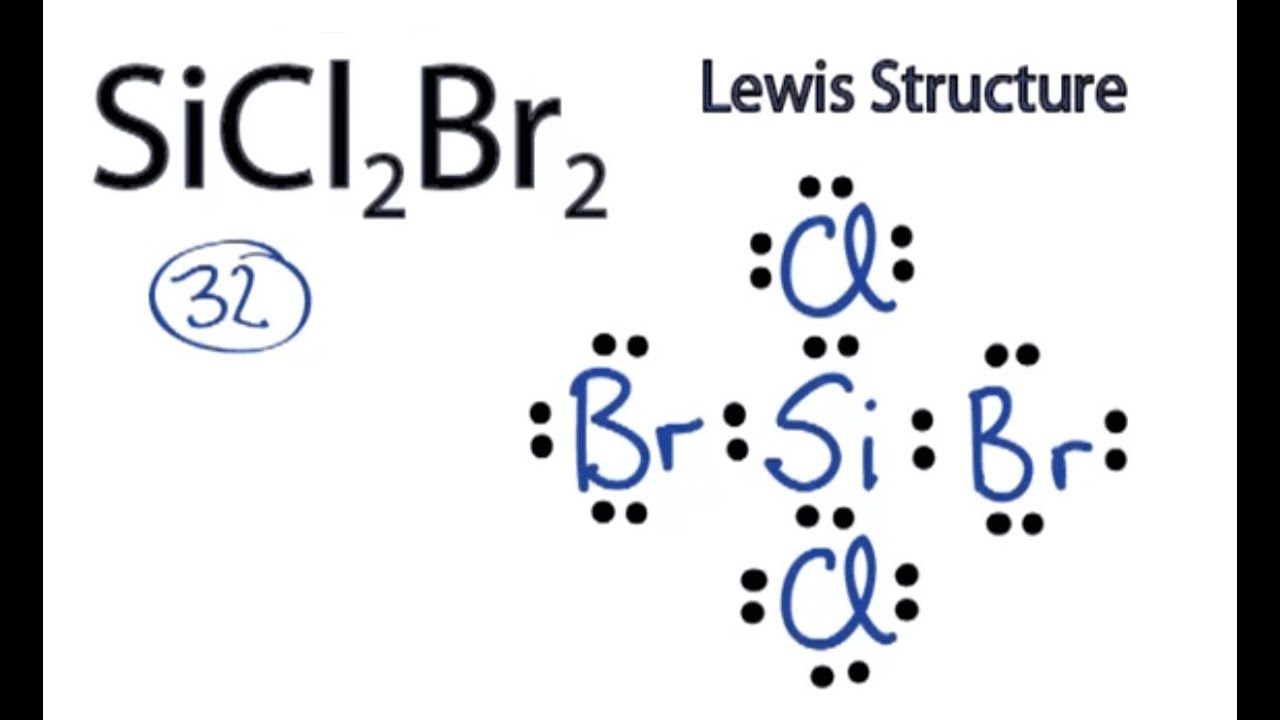
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
Solved Draw the electrondot formula for SiCl2Br2. To add
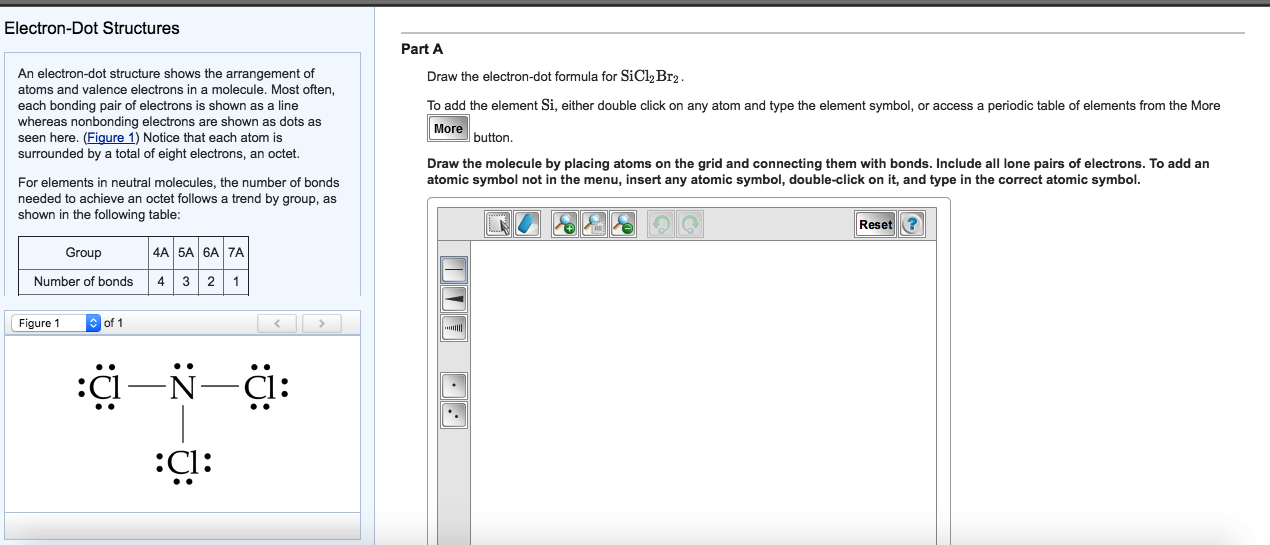
Solved Draw the electrondot formula for SiCl2Br2. To add
lewis structure for sicl2br2

Draw the Lewis Structure for Sicl2br2.
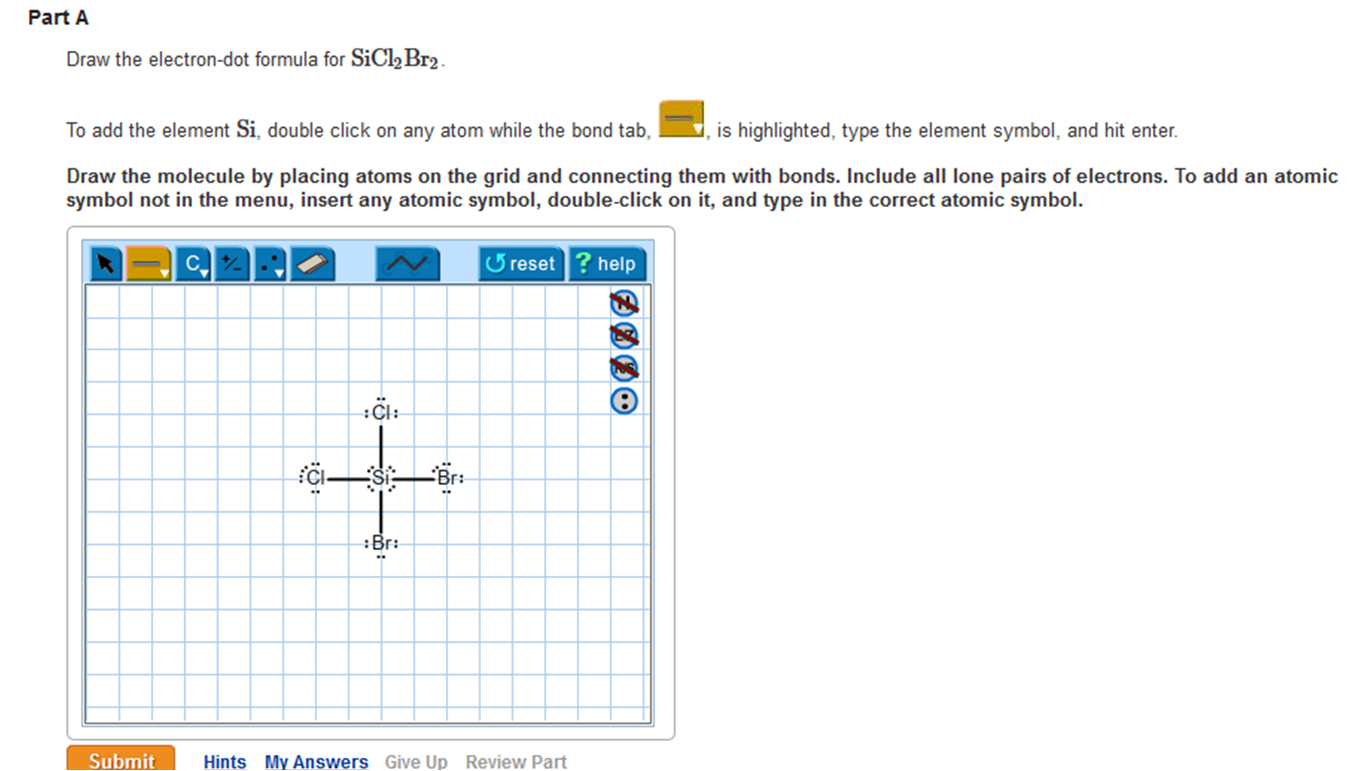
Solved Draw the electrondot formula for SiCl2Br2. This is
lewis structure for sicl2br2
1 Become A Study.com Member To Unlock This Answer!
Web How Do You Draw A Lewis Structure For Sicl_2Br_2?
Expert Answer 100% (72 Ratings)
Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
Related Post: