Draw The Lewis Structure For N2
Draw The Lewis Structure For N2 - Web each nitrogen atom has five valence electrons. For the n2 structure use the periodic table to find the total number of valence. Send feedback | visit wolfram|alpha get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find total number of electrons of the valance shells of nitrogen atoms determine total electrons pairs existing as lone pairs and bonds Since there are two nitrogen atoms in n 2 you have a total of ten valence electrons to work with. The lewis structure helps us visualize the arrangement of atoms and electrons in a molecule. Put the least electronegative atom in the center. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. Web here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen molecule. Web in this video i will show you the fundamentals on how to draw a lewis dot structure as while showing you how to draw the lewis dot structure for n2. Web added jun 9, 2014 by webtester in chemistry this widget gets the lewis structure of chemical compounds. Web this is the total number of electrons that must be used in the lewis structure. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are. Web lewis structures for covalent molecules: Drawing lewis structures for molecules with one central atom: Follow along using the transcript. Calculate the total number of valence electrons. Since n is a member of the group 5a (based on the periodic table), the number of electrons in its outermost shell must be 5. Web here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen molecule. While selecting the atom, you have to put the least electronegative atom at the center. Web to draw the n2 lewis structure, you can follow these steps: Web representing a covalent bond using lewis structures. Find total number of electrons of the valance shells of. Since there are two nitrogen atoms in n 2 you have a total of ten valence electrons to work with. Web because n 2 is a simple molecule, it is extremely easy to draw the lewis structure of it. Web this is the total number of electrons that must be used in the lewis structure. The lewis structure indicates the. For the n2 structure use the periodic table to find the total number of valence. Here is the electron dot structure for a single n atom: The lewis diagram for n 2 is as follows: Web to draw the n2 lewis structure, you can follow these steps: The lewis diagram that fills each atom’s valence electron shell is as follows: While selecting the atom, you have to put the least electronegative atom at the center. Web 17k views 2 years ago. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. In any molecule or ion with the general. Here is the electron dot structure for a single n atom: Determine the central atom in n2, both nitrogen atoms are equivalent, so there is no distinct. Find the total valence electrons for the n2 molecule. Web 17k views 2 years ago. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen. The atomic number of nitrogen is 7 having an electronic configuration of 1s 2, 2s 2, 2p 3. Web the first step is to sketch the lewis structure of the n2 molecule, to add valence electrons around the two nitrogen atoms, and the final step is to combine the two nitrogen diatomic atoms to get the n2 lewis structure. Web. Web this is the total number of electrons that must be used in the lewis structure. Generally, small symmetric molecules are nonpolar. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons in the molecule. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. However. The lewis structure indicates the atom and its position in the model of the molecule using its. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. Web steps to properly draw the n 2 lewis structure, follow these steps: Web in this video i will show you the fundamentals on how to draw a lewis. Web to draw the n2 lewis structure, you can follow these steps: Since there are two nitrogen atoms in n 2 you have a total of ten valence electrons to work with. While selecting the atom, you have to put the least electronegative atom at the center. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. Rules to draw lewis structure. The lewis structure helps us visualize the arrangement of atoms and electrons in a molecule. Follow along using the transcript. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. Firstly, check out the atomic number of each atom from the periodic table. Generally, small symmetric molecules are nonpolar. Find the total valence electrons for the n2 molecule. Web this is the total number of electrons that must be used in the lewis structure. Remember, valence electrons are those in the outermost principal energy level. Therefore, n 2 is a nonpolar substance. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary #4 minimize formal charges by converting lone pairs of the atoms #5 repeat step 4 if necessary, until all charges are minimized Web added jun 9, 2014 by webtester in chemistry this widget gets the lewis structure of chemical compounds.
Lewis Dot Structure For N2 Draw Easy

N2 Lewis Structure How To Draw The Lewis Structure For N2
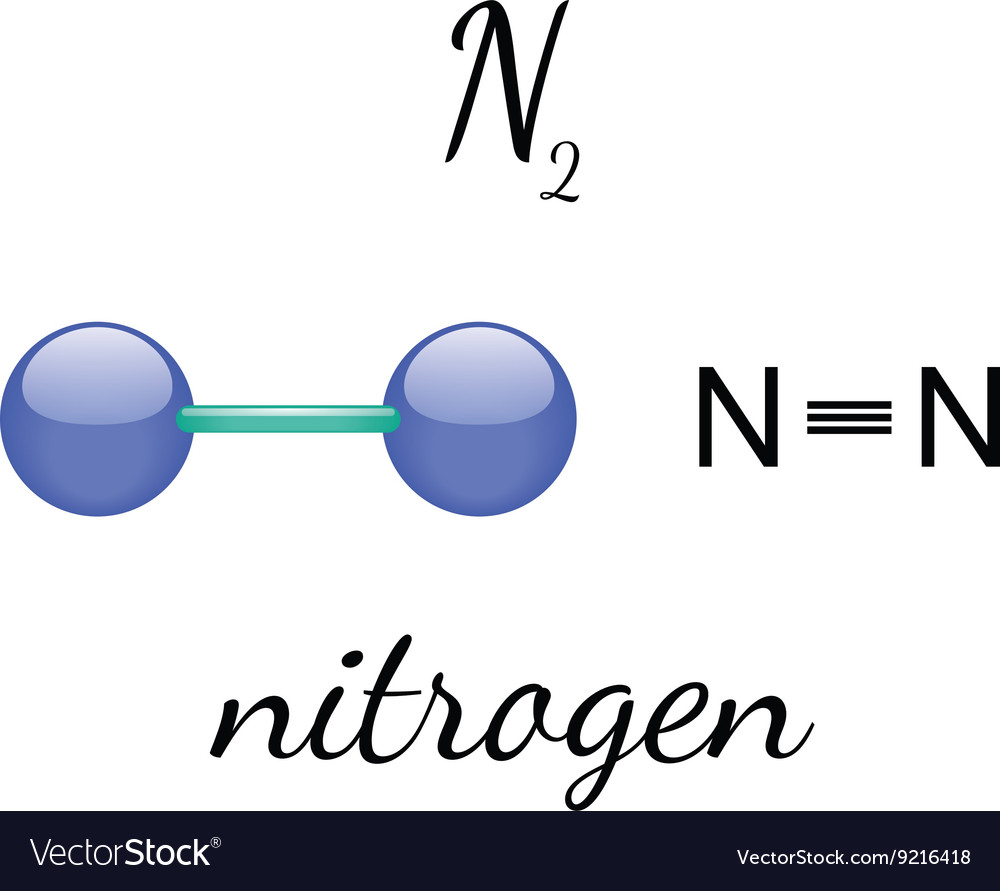
N2 nitrogen molecule Royalty Free Vector Image
Lewis Electron Dot Diagram Of N2 slide share
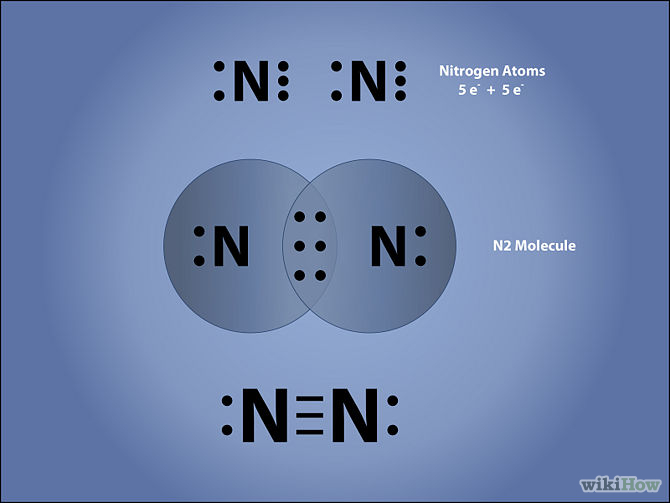
Lewis Structure for N2 How To Discuss
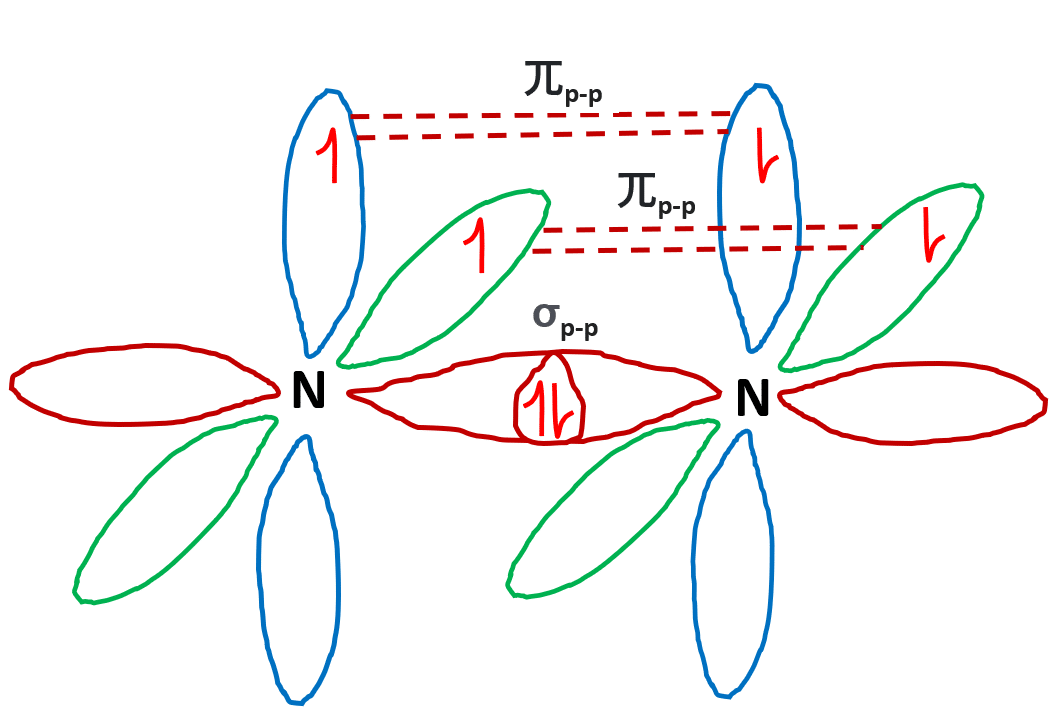
N2 Lewis Structure Hybridization & Molecular Geometry What's Insight

N2 Lewis Structure How to Draw the Lewis Structure for N2 Nitrogen Gas

How to draw N2 Lewis Structure Science Education and Tutorials
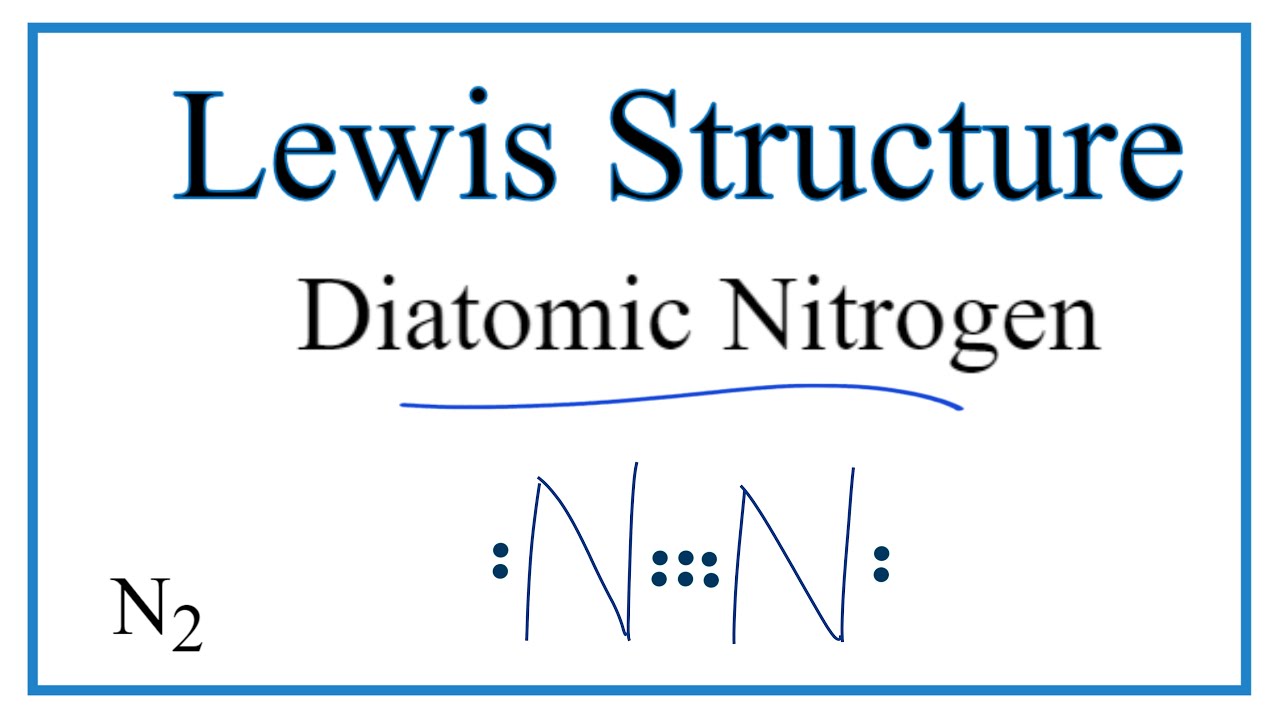
How to Draw the Lewis Dot Structure for Diatomic Nitrogen (N2) YouTube

What is the Lewis structure of N2? Socratic
Calculate The Total Number Of Valence Electrons.
Find Total Number Of Electrons Of The Valance Shells Of Nitrogen Atoms Determine Total Electrons Pairs Existing As Lone Pairs And Bonds
The First Thing We Need To Do When Drawing A Lewis Structure Is Determine The Total Number Of Valence Electrons In The Molecule.
Web 17K Views 2 Years Ago.
Related Post:
