Draw The Lewis Structure For Scl2
Draw The Lewis Structure For Scl2 - You'll get a detailed solution from a subject matter expert that. The chemical formula scl2 represents sulfur dichloride. This problem has been solved! The second step is to add valence electrons to the two chlorine atoms, and the final step is to combine the. Use your molecular models to explain why this molecule is polar. Web scl2 lewis structure, molecular geometry, hybridization, bond angle and shape. Web to properly draw the scl 2 lewis structure, follow these steps: The following procedure can be used to construct lewis electron structures for simple molecules. Chlorine, group 7 or 17 has 7; Sulfur is in group 6 of the periodic table , so it has 6 valence electrons. Scl2 is used to synthesize. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. I can't draw it but each atom has 6 electrons around it. Web 6 steps to draw the lewis structure of scl2 step #1: Draw the lewis electron dot structure for scl2 and discuss its molecular. You'll get a detailed solution from a subject matter expert that. [ ] the molecule is nonpolar. The second step is to add valence electrons to the two chlorine atoms, and the final step is to combine the. Written by priyanka in lewis structure. Web watch on steps of drawing scl2 lewis structure step 1: The second step is to add valence electrons to the two chlorine atoms, and the final step is to combine the. Drawing the lewis structure for scl2 Web 6 steps to draw the lewis structure of scl2 step #1: Web to properly draw the scl 2 lewis structure, follow these steps: Web watch on steps of drawing scl2 lewis structure. Use your molecular models to explain why this molecule is polar. Web steps of drawing lewis structure of scl 2 when we draw lewis structures, there are several guidelines to follow. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Sulfur is in group 6 of the periodic table , so it has. Web sulfur is the central atom in the lewis structure of scl2 that has a steric number equal to 4,. Web watch on see the big list of lewis structures transcript: [ ] the molecule is nonpolar. Let's do the scl2 lewis structure. Chlorine, group 7 or 17 has 7; Web by using the following steps, you can easily draw the lewis structure of scl 2: #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. Web to properly draw the scl 2 lewis structure, follow these. Web sulfur is the central atom in the lewis structure of scl2 that has a steric number equal to 4,. Calculate the total number of valence electrons. Web draw lewis structures for covalent compounds. Web scl2 is unstable and exists in equilibrium with s2cl2, as shown. Number of steps can be changed according the complexity of the molecule or ion. (b) what is the the shape (molecular geometry) of scl2? The following procedure can be used to construct lewis electron structures for simple molecules. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web watch on see the big list of lewis structures transcript: Chlorine, group 7 or 17 has. Web by using the following steps, you can easily draw the lewis structure of scl 2: #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. Scl2 is used to synthesize. Find the total valence electrons in. [ ] the molecule is nonpolar. Sulfur is in group 6 of the periodic table , so it has 6 valence electrons. Six plus 14 equals 20 valence electrons. Chlorine is in group 7, so it has 7 valence electrons. Web lewis dot structure of scl2 (sulfur dichloride) i quickly take you through how to draw the lewis structure of. This problem has been solved! Web steps of drawing lewis structure of scl 2 when we draw lewis structures, there are several guidelines to follow. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Chlorine is in group 7, so it has 7 valence electrons. Let's do the scl2 lewis structure. Written by priyanka in lewis structure. Calculate the total number of valence electrons. It is obtained via chlorination of s2cl2 whose impure presence is then. Web drawing lewis structures for molecules with one central atom: While selecting the atom, always put the least electronegative atom at the center. Web to properly draw the scl 2 lewis structure, follow these steps: I can't draw it but each atom has 6 electrons around it. Use your molecular models to explain why this molecule is polar. This problem has been solved! Web scl2 is unstable and exists in equilibrium with s2cl2, as shown. Web to draw the lewis structure for scl2 (sulfur dichloride), we need to determine the valence electrons in scl2.
SCl2 Lewis Structure, Geometry, Hybridization, and Polarity

Lewis Structure of SCl2 (sulfur dichloride) YouTube

So far, we’ve used 20 of the SCl2 Lewis structure’s total 20 outermost
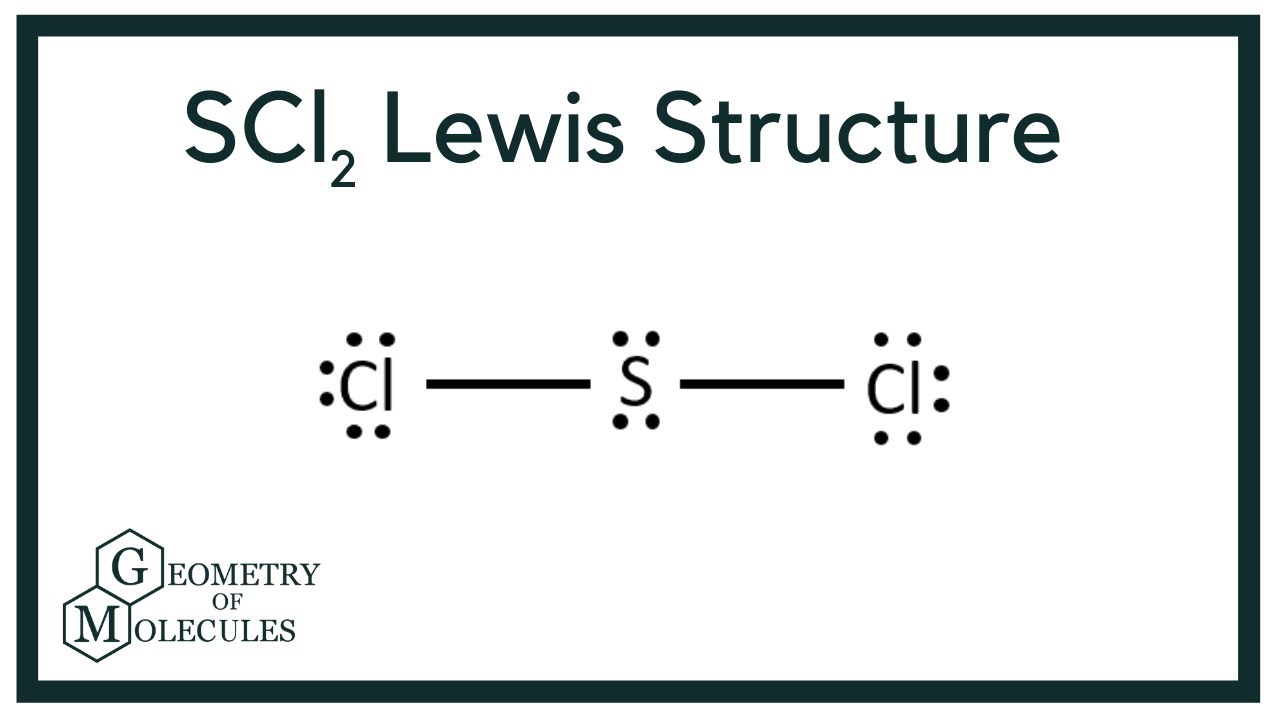
Lewis Dot Structure For Scl2 Draw Easy

SCl2 (Sulfur dichloride) Molecular Geometry, Bond Angles & Electron

SCl2 Molecular Geometry Science Education and Tutorials
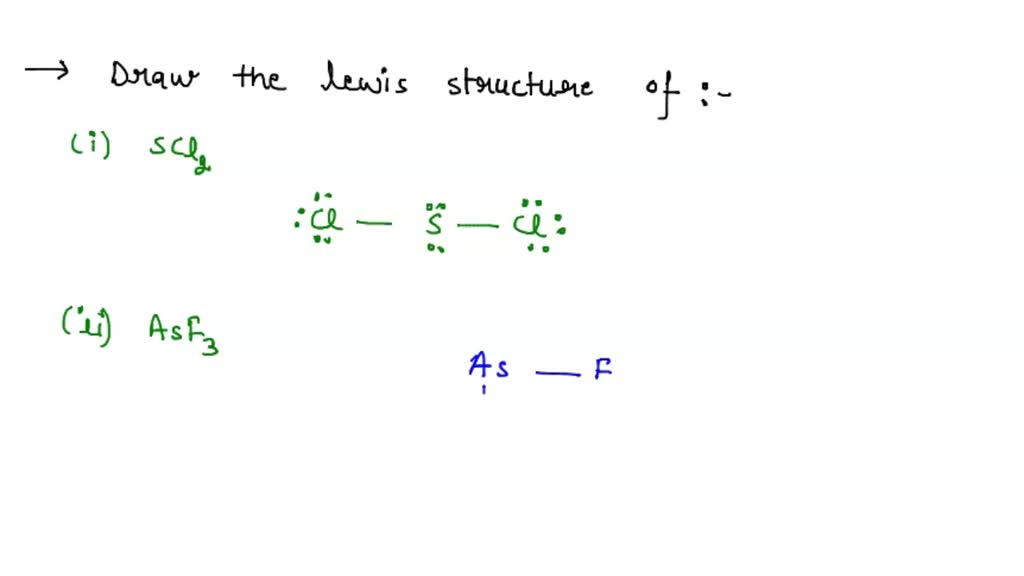
SOLVED 1) Draw the Lewis structure of A) SCl2 B) AsFr3 2) Using only

SOLVED Draw the Lewis structure for SCl2. How many lone pairs of

Is SCl2 Polar or Nonpolar? Techiescientist
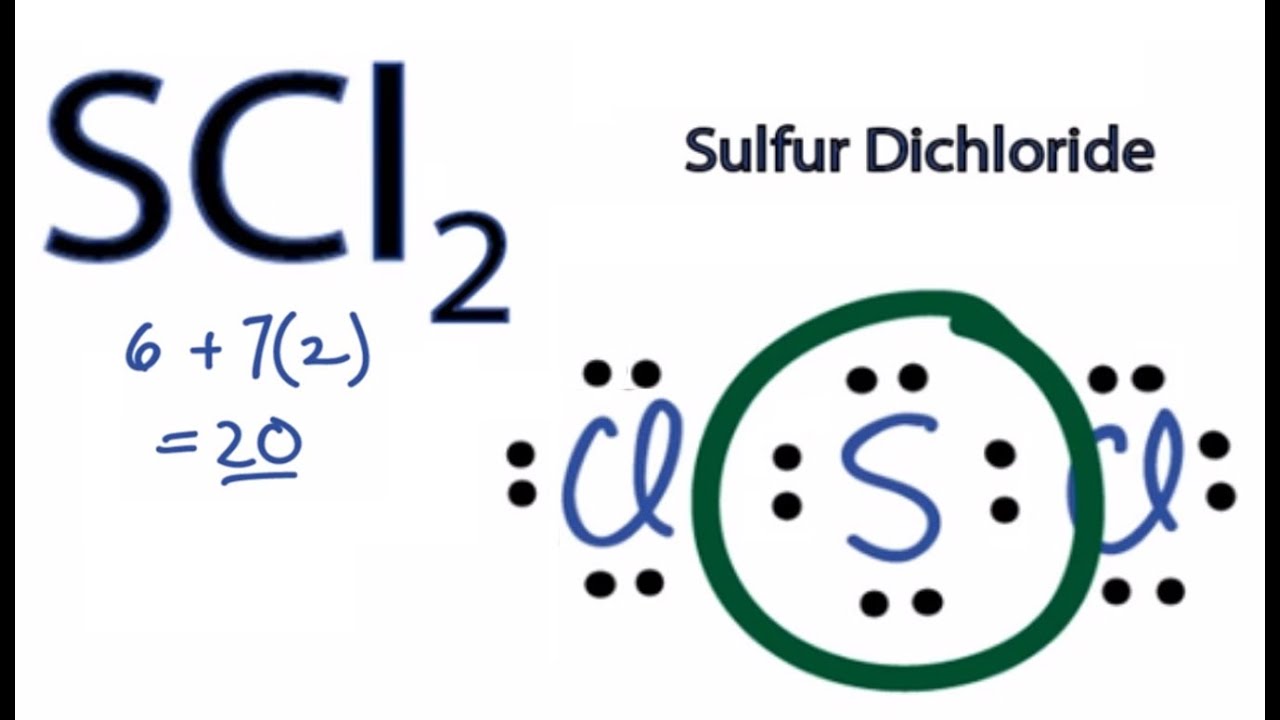
SCl2 Lewis Structure How to Draw the Lewis Structure for SCl2 YouTube
Chlorine, Group 7 Or 17 Has 7;
You'll Get A Detailed Solution From A Subject Matter Expert That.
This Means They Will Share Electrons To Form Covalent Bonds.sulfur Shares One Electron With Each Of The Two Chlorin.
Sulfur Is In Group 6 Of The Periodic Table , So It Has 6 Valence Electrons.
Related Post: