Draw The Lewis Structure For Sf2
Draw The Lewis Structure For Sf2 - For drawing the sf2 lewis structure it is important to know the number of valence electrons present in the compound. Web the main objective of the question is to draw the lewis structure for sf a 2 along with its molecular geom. Let's do the sf2 lewis structure. Web chemistry 360 14.9k subscribers subscribe share 2.2k views 3 years ago playlist:lewis structure (click here to learn more) sf2 lewis structure||lewis structure of sf2 (sulfur. #1 draw a rough skeleton structure first, determine the total number of valence electrons What is the approximate bond angle in sf2? Web in this comprehensive guide, we'll walk you through the process of drawing the lewis structure for sf2, sulfur difluoride. Fluorine has 7, but we have two of them. Calculate the total number of valence electrons. What is the hybridization of the central atom? Web draw the best lewis structure for sf2, including all nonbonding electrons and all nonzero formal charges if applicable. Web 6 steps to draw the lewis structure of sf2 step #1: The lewis structure of sf2 contains 16 nonbonding electrons and 4 bonding electrons. Include all lone pairs of electrons. Select a tool to begin drawing < 0506 macbook pro. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. To determine the total number of valence electrons in sf2, we need to. Here let’s see how to draw it. Find the total valence electrons in sf2. Draw the lewis structure of sf2 showing all lone pairs. This problem has been solved! Draw the lewis structure of sf2, showing all lone pairs. Web drawing lewis structures for molecules with one central atom: Web science chemistry chemistry questions and answers draw the lewis structure of sf2. The second step is to add valence electrons to the two fluorine atoms, and the final step is to combine the. Web how to draw the lewis structure for sf2? Web how to draw lewis structure of sf2 step 1: Web here’s how you can easily draw the sf 2 lewis structure step by step: The lewis structure of sf2 is similar to the sbr2 and scl2. Let's do the sf2 lewis structure. Identify the molecular geometry of sf2. Web chemistry questions and answers. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Find the total valence electrons in sf2. Put the least electronegative atom in the center. It is important to look at what the lewis structure of sf2 is so that we can move ahead and look at other aspects of it. Select a tool to begin drawing < 0506 macbook pro 36 3む procasemand this problem has been solved! While selecting the atom, always put the least. Before we dive into drawing the lewis structure of sf2, it's crucial to gather some essential information about the molecule. The second step is to add valence electrons to the two fluorine atoms, and the final step is to combine the. Web lewis structure is nothing but an arrangement of valence electrons between different atoms. Here, the given molecule is. Find the total valence electrons in sf2 molecule in order to find the total valence electrons in sf2 (sulfur difluoride) molecule, first of all you should know the valence electrons present in sulfur atom as well as fluorine atom. Web here’s how you can easily draw the sf 2 lewis structure step by step: Web the first step is to. Web chemistry 360 14.9k subscribers subscribe share 2.2k views 3 years ago playlist:lewis structure (click here to learn more) sf2 lewis structure||lewis structure of sf2 (sulfur. The lewis structure of sf2 is similar to the sbr2 and scl2. Web sf2 lewis structure is made up of two atoms, sulfur (s), and fluorine (f), the sulfur (s) is in the central. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web chemistry questions and answers. To determine the total number of valence electrons in sf2, we need to. Web sf2 lewis structure is made up of two atoms, sulfur (s), and fluorine (f), the sulfur (s) is in the. Here let’s see how to draw it. What is the approximate bond angle in sf2? Web here’s how you can easily draw the sf 2 lewis structure step by step: The second step is to add valence electrons to the two fluorine atoms, and the final step is to combine the. Here sulphur (atomic number = 16 and electronic configuration = 2,8,6) has 6 valence electrons. Web lewis structure is nothing but an arrangement of valence electrons between different atoms. Put the least electronegative atom in the center. Identify the molecular geometry of sf2. It is important to look at what the lewis structure of sf2 is so that we can move ahead and look at other aspects of it. The lewis structure of sf2 is similar to the sbr2 and scl2. Here, the given molecule is sf2 (sulfur difluoride). This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Calculate the total number of valence electrons. Select a tool to begin drawing < 0506 macbook pro 36 3む procasemand this problem has been solved! Web the first step is to sketch the lewis structure of the sf2 molecule, to add valence electrons around the sulfur atom;
Draw the lewis structure of sf2 showing all lone pairs punchstart
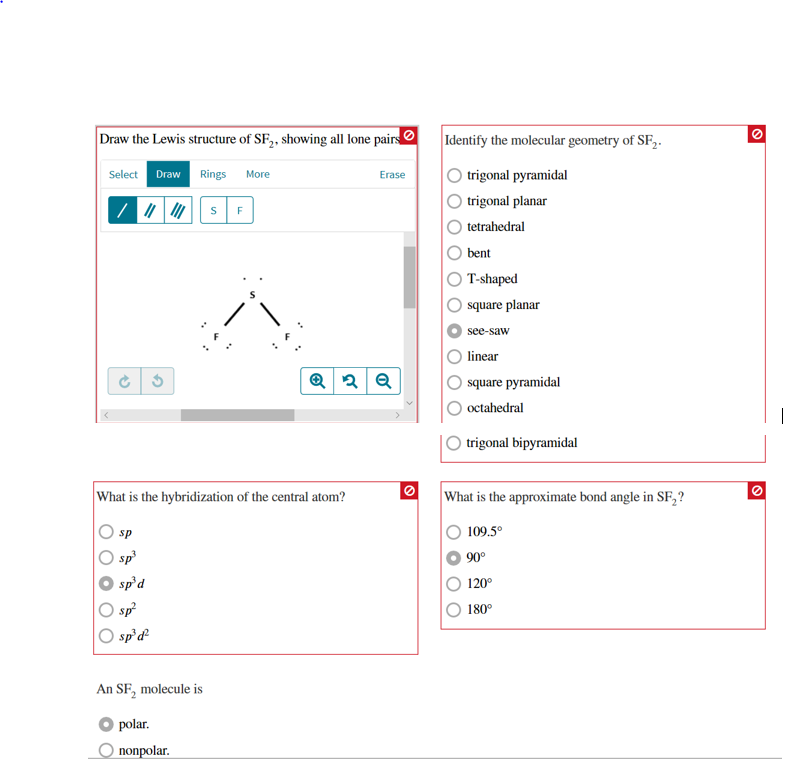
Draw the lewis structure of sf2 showing all lone pairs torbucket
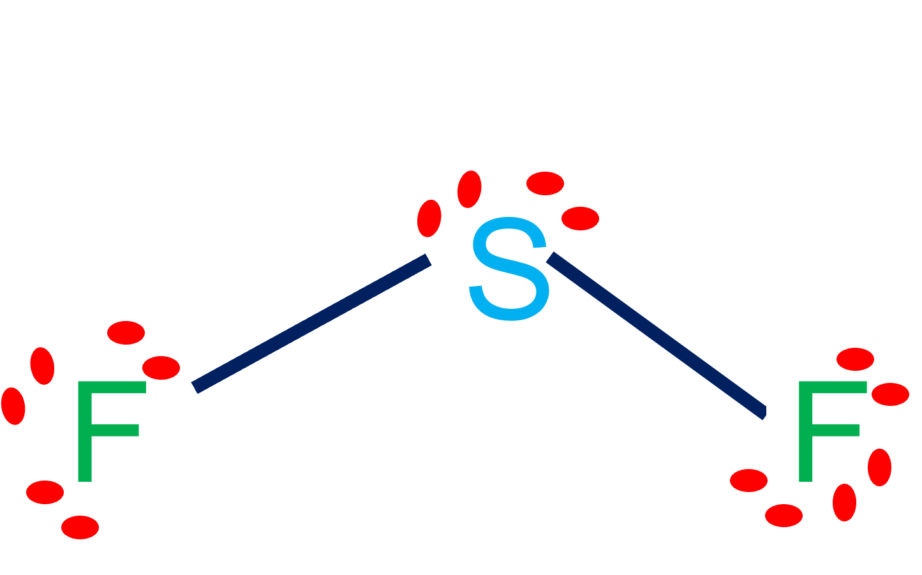
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight

Draw the Lewis structure of SF2, showing all lone pairs. Identify the
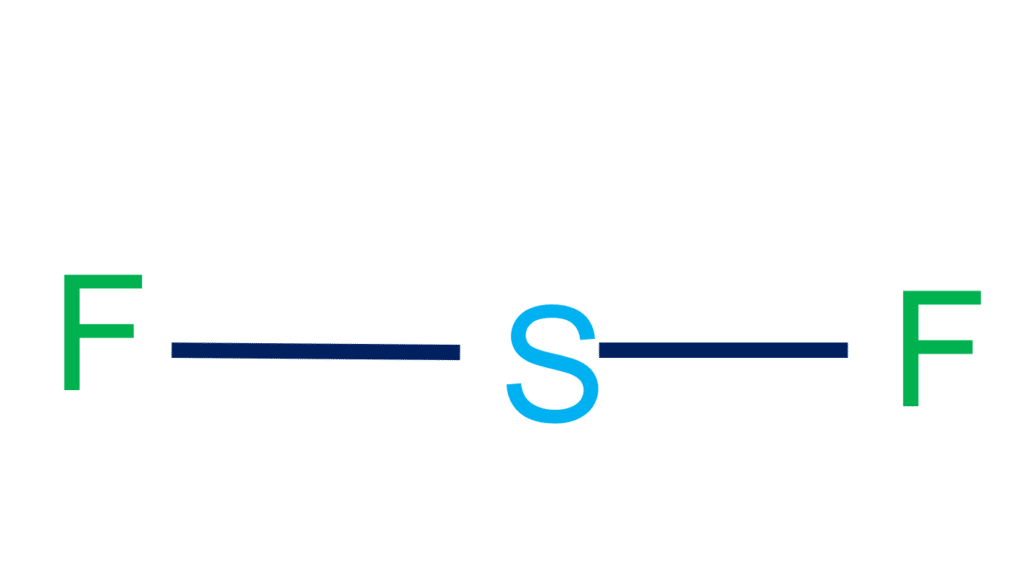
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight

So far, we’ve used 20 of the SF2 Lewis structure’s total 20 outermost

How to draw SF2 Lewis Structure? Science Education and Tutorials

SF2 Lewis Structure How to Draw the Lewis Structure for SF2 YouTube

SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and

SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
Find The Total Valence Electrons In Sf2.
While Selecting The Atom, Always Put The Least Electronegative Atom At The Center.
Web Sf2 Lewis Structure Is Made Up Of Two Atoms, Sulfur (S), And Fluorine (F), The Sulfur (S) Is In The Central Position And Fluorine (F) Atoms Are On Either Side Of It.
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
Related Post: