Draw The Lewis Structure For Sif4
Draw The Lewis Structure For Sif4 - Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. So we have 32 valence electrons to work with. Web chemistry questions and answers. In a silicon tetrafluoride molecule, the silicon atom and four fluorine atoms together form a sif4 covalent compound. Draw the molecule by placing atoms on the grid and connecting them with bonds. Here, the given molecule is sif4 (silicon tetrafluoride). Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Web to properly draw the sif 4 lewis structure, follow these steps: Web by using the following steps, you can easily draw the lewis structure of sif 4: 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. Calculate the total number of valence electrons. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. Web in this article, we are going to discuss. Web chemistry chemistry questions and answers 1. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Web a video explanation of how to draw the lewis dot structure for silicon tetrafluoride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and. Web to draw the sif4. And that is 4 plus 28, is 32. Here’s the best way to solve it. Web by using the following steps, you can easily draw the lewis structure of sif 4: For the sih4 structure use the periodic table to find the total number of valence electrons. Web science chemistry chemistry questions and answers question 25 (1 point) draw the. For the sih4 structure use the periodic table to find the total number of valence electrons. Web steps of drawing sif4 lewis structure step 1: Web chemistry chemistry questions and answers 1. (c) cof2 (c is central) show transcribed image text. Six electrons are used, and 6 are left over. Web the first step is to sketch the molecular geometry of the sif4 molecule, to calculate the lone pairs of the electron in the central silicon atom; And that is 4 plus 28, is 32. Part b draw the lewis structure for co. Web part a draw the lewis structure for sih4. Web in this article,”sif4 lewis structure”, different facts. Web by using the following steps, you can easily draw the lewis structure of sif 4: Web in this article, we are going to discuss the chemical bonding of silicon tetrafluoride by understanding its lewis structure, molecular geometry, and the hybridization of the central atom. Web a video explanation of how to draw the lewis dot structure for silicon tetrafluoride,. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the molecule by placing atoms on the grid and connecting them with bonds. Draw a lewis structure for (a) sif4; Web the first step is to sketch the molecular geometry of the sif4 molecule, to calculate the lone pairs of the electron. Web to properly draw the sif 4 lewis structure, follow these steps: So we have 32 valence electrons to work with. In a silicon tetrafluoride molecule, the silicon atom and four fluorine atoms together form a sif4 covalent compound. See examples of polar molecules. Determine the total number of valence electrons in the molecule by adding up the valence electrons. Web part a draw the lewis structure for sih4. Web chemistry chemistry questions and answers 1. 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. Web a video explanation of how to draw the lewis dot structure for silicon tetrafluoride, along with information about the compound including formal charges,. Web 6 steps to draw the lewis structure of sif4 step #1: A = 5 in., b = 12 in. Web steps of drawing sif4 lewis structure step 1: Then, we will study the polarity of sif4 i.e., whether sif4 is a polar or nonpolar molecule. Include all lone pairs of electrons. Web a video explanation of how to draw the lewis dot structure for silicon tetrafluoride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and. Web part a draw the lewis structure for sih4. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. Related to this question for sf3+,. Web how to draw lewis structure for sif4 silicon tetrafluoridelewis structure: So we have 32 valence electrons to work with. The second step is to valence electron to the fluorine atom, and the final step is to combine the step1 and step2 to get the ccl4 lewis structure. Calculate the total number of valence electrons. Then, we will study the polarity of sif4 i.e., whether sif4 is a polar or nonpolar molecule. First, draw a lewis structure for sif4. Web in this article,”sif4 lewis structure”, different facts like lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. Web in this article, we are going to discuss the chemical bonding of silicon tetrafluoride by understanding its lewis structure, molecular geometry, and the hybridization of the central atom. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. On the periodic table, silicon is in group 4, sometimes called 14, so it's got 4 valence electrons. Web to draw the sif4 lewis structure, follow these steps: For the sih4 structure use the periodic table to find the total number of valence electrons.
Sif4 Lewis Structure
Sif4 Lewis Structure
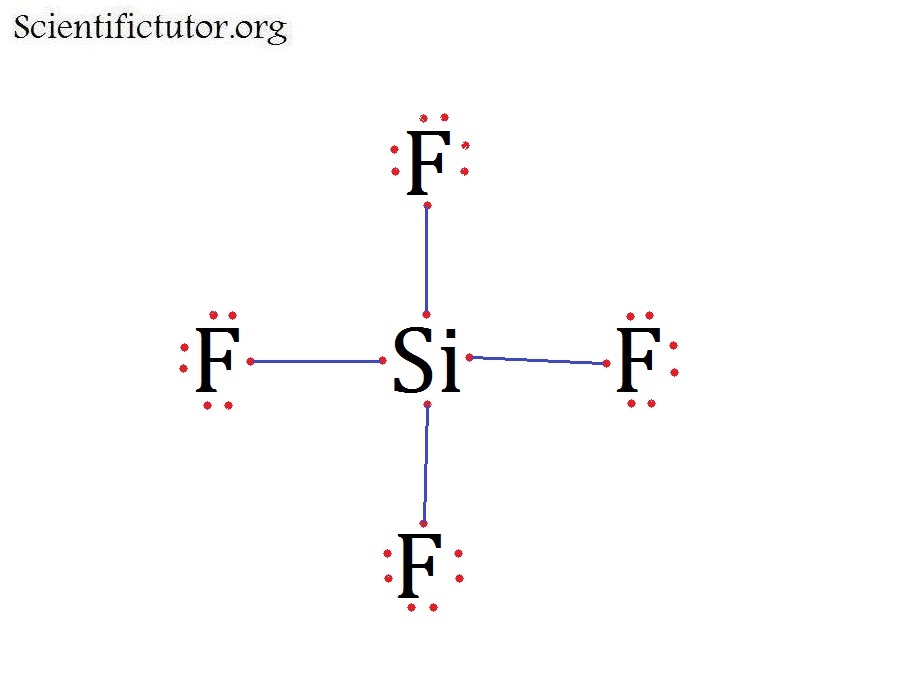
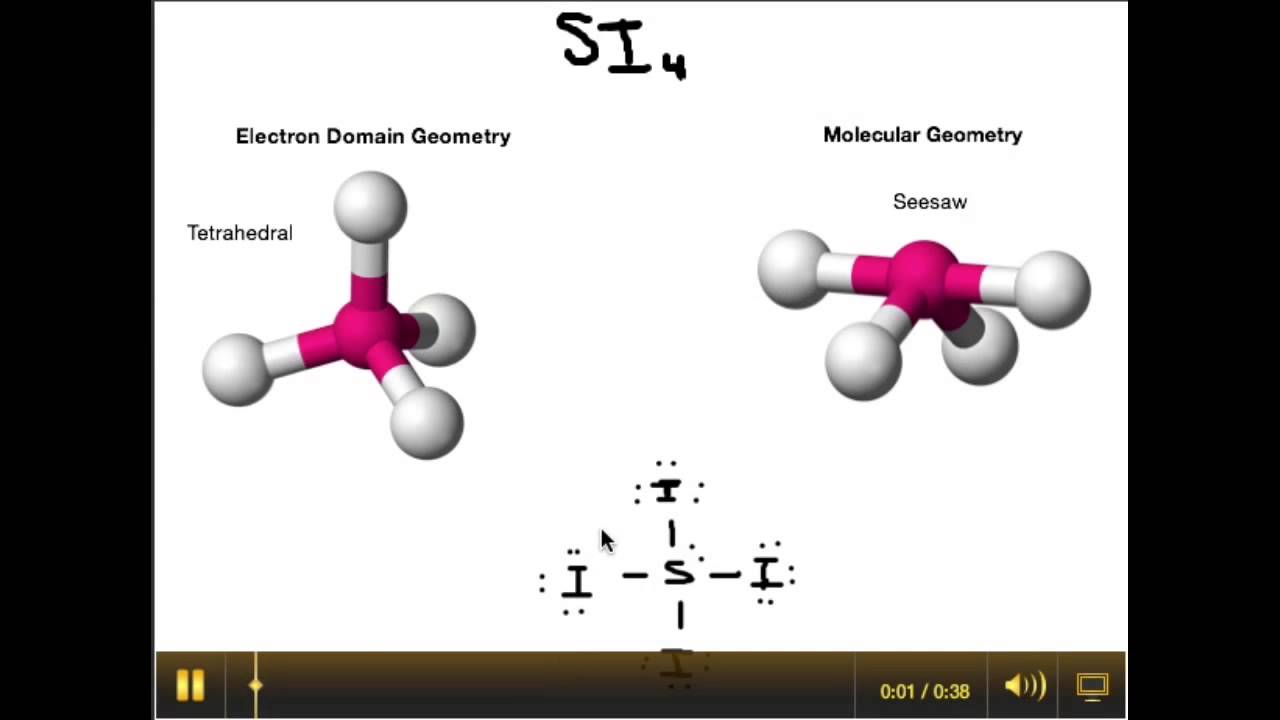
Chem Molecular Shape (Molecular Geometry) Scientific Tutor

Draw The Lewis Structure For Sif4 Drawing Easy
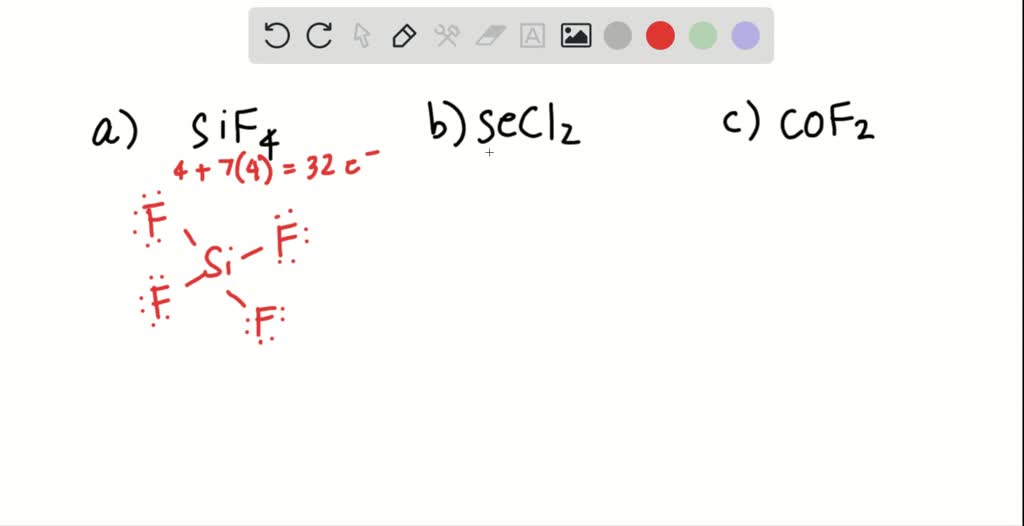
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is

How to draw SiF4 Lewis Structure? Science Education and Tutorials
Lewis Structure For Sif4

SiF4 Lewis Structure How to Draw the Dot Structure for SiF4 YouTube

Lewis Structure For Sif4
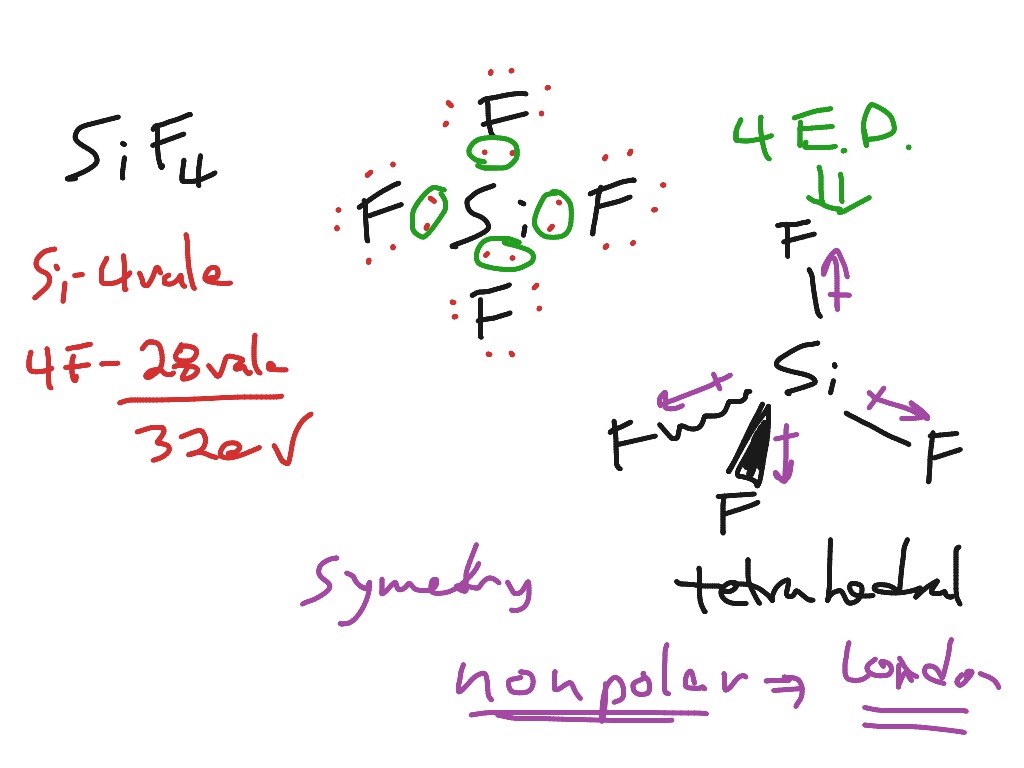
Sif4 Lewis Structure
Here, The Given Molecule Is Sif4 (Silicon Tetrafluoride).
Web Science Chemistry Chemistry Questions And Answers Question 25 (1 Point) Draw The Lewis Structure For Sif4;
Placing A Bonding Pair Of Electrons Between Each Pair Of Bonded Atoms Gives The Following:
Each Hydrogen Atom (Group 1) Has One Valence Electron, Carbon (Group 14) Has 4 Valence Electrons, And Oxygen (Group 16) Has 6 Valence Electrons, For A Total Of [ (2) (1) + 4 + 6] = 12 Valence Electrons.
Related Post: