Draw The Lewis Structure For The Iodide Pentafluoride Molecule
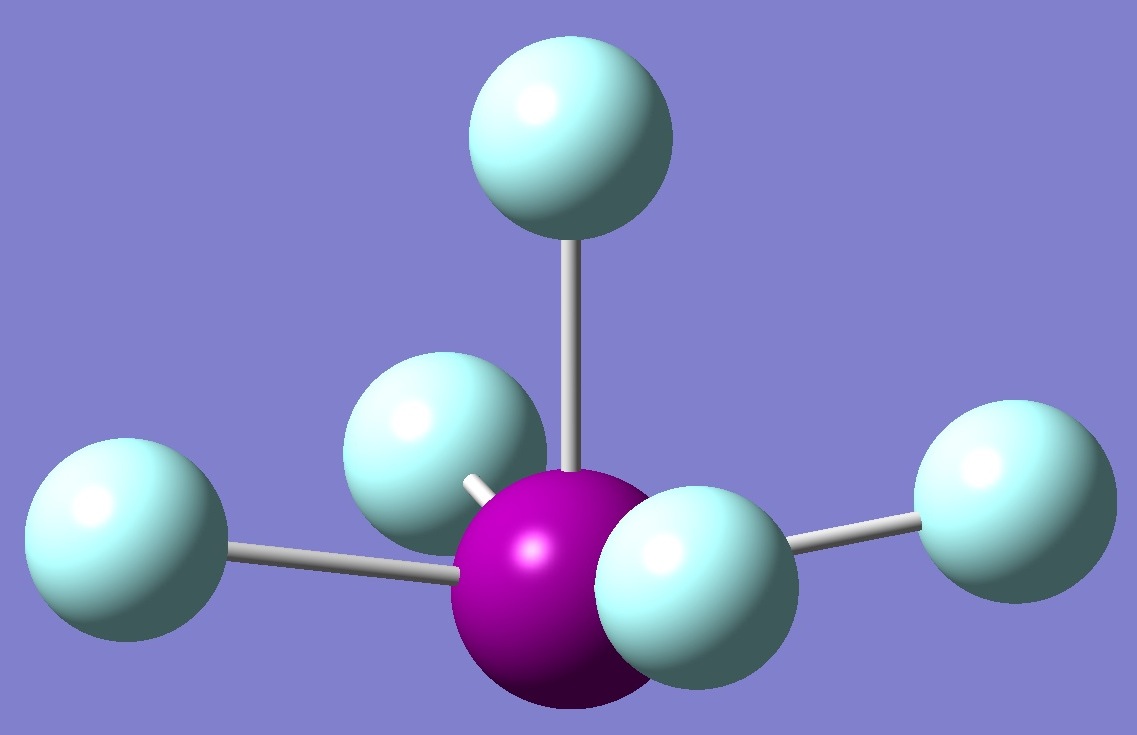
Draw The Lewis Structure For The Iodide Pentafluoride Molecule - Draw the lewis structure for the iodide pentafluoride (if:) molecule. Web iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1 is the elected configuration of hydrogen. Ć ro | с | х 5 ? Web in this question, we have to draw the lewis dot structure of hydrogen. (c) which is the more polar bond. Web drawing lewis structures for molecules with one central atom: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Assign an ax m e n designation; There is an electronic configuration of kr four d 10, five s two, and b five. This problem has been solved! The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. 1 shows the lewis symbols for the elements of the third period of the periodic table. This means that iodine has seven valence. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. We have to draw the lewis dot structure of hydrogen. Web in this question, we have to draw the lewis dot structure of hydrogen. Web write the lewis structure for nitrosyl fluoride, fno. Find more chemistry widgets in wolfram|alpha. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web chemistry chemistry questions and answers draw the lewis structure for the iodide pentafluoride (if:) molecule. This problem has been solved! Here’s the best way to solve it. Find more chemistry widgets in wolfram|alpha. Web science chemistry chemistry questions and answers draw the lewis structure for the iodide pentafluoride molecule. Web drawing lewis structures for molecules with one central atom: Draw the lewis structure for the iodide pentafluoride (if:) molecule. Web a video explanation of how to draw the lewis dot structure for iodine pentafluoride, along with information about the compound including formal charges,. 1 shows the lewis symbols for the elements of the third period of the periodic table. Lewis dot structure of if5 (iodine pentafluoride) watch on. Experts have been vetted by chegg as specialists in this subject. Web science chemistry chemistry questions and answers draw the lewis structure for the iodide pentafluoride molecule. Web chemistry chemistry questions and answers draw the. There is an electronic configuration of kr four d 10, five s two and b five. Draw the lewis structure of chlorine pentafluoride. Web the lewis structure helps us identify the bond pairs and the lone pairs. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Web chemistry chemistry questions and answers draw. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Assign an ax m e n designation; Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Using only. Determine the electron group arrangement around the central atom that minimizes repulsions. Web chemistry chemistry questions and answers draw the lewis structure for the iodide pentafluoride (if:) molecule. Why iodine has the electronic configuration of kr four d 10, five s two by b five. Using only a periodic table, identify (a) which is the longer bond. Web science chemistry. 1 is the elected configuration of hydrogen. We have to draw the lewis dot structure of hydrogen. Iodide is what it is. Web © 2023 google llc hello, it's time for your daily chemistry dose! Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The structure on the right is the lewis electron structure, or lewis structure, for h 2 o. Web the lewis structure helps us identify the bond pairs and the lone pairs. There is an electronic configuration of kr four d 10, five s two and b five. Here’s the best way to solve it. This means that iodine has seven. Hydrogen has the elected configuration of one s. There is an electronic configuration of kr four d 10, five s two and b five. Web draw the lewis structure for the chlorine pentafluoride (clf5) molecule. 1 has the elected configuration of hydrogen. Web chemistry chemistry questions and answers draw the lewis structure for the iodide pentafluoride (if:) molecule. Draw the lewis structure for the iodide pentafluoride (if:) molecule. Web this problem has been solved! Iodide is what it is. Ć ro | с | х 5 ? Lewis dot structure of if5 (iodine pentafluoride) watch on. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. This gives it ax5e1 shape by vsepr, and square pyramidal molecular. Determine the total number of valence (outer shell) electrons. Web iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. Web drawing lewis structures for molecules with one central atom: Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:
Lewis Structure For Iodine
Iodine dot structure 🍓ICl Lewis Structure How to Draw the Lewis
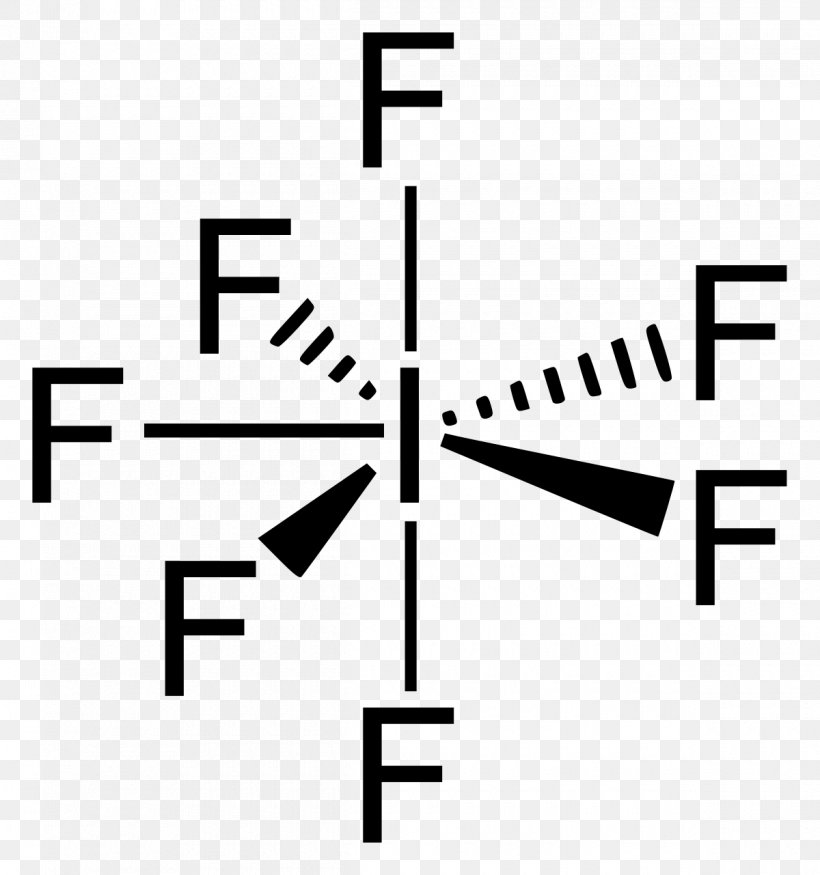
Lewis Structure Iodine Pentafluoride Lewis Acids And Bases Molecular
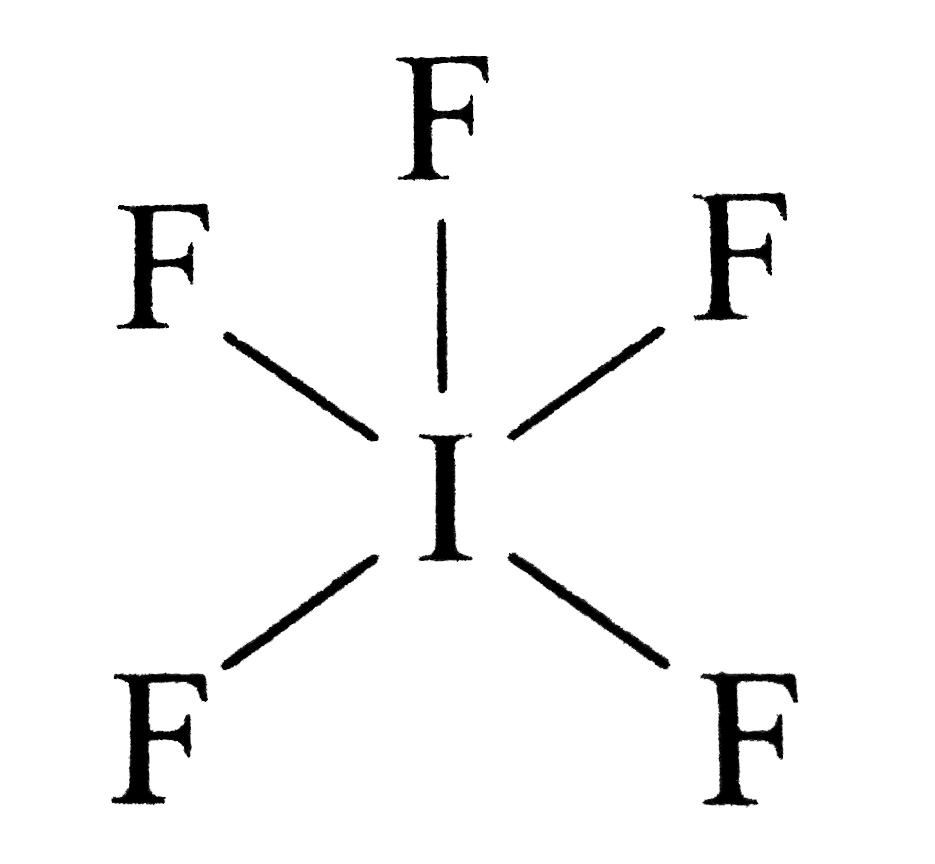
Draw the Lewis structure of iodine pentafluoride, IF5.

SOLVED Draw the Lewis structure for the iodide pentafluoride (TFs

Iodine pentafluoride lewis structure YouTube
IF5 Lewis Structure (Iodine Pentafluoride) IF5 Lewis Structure
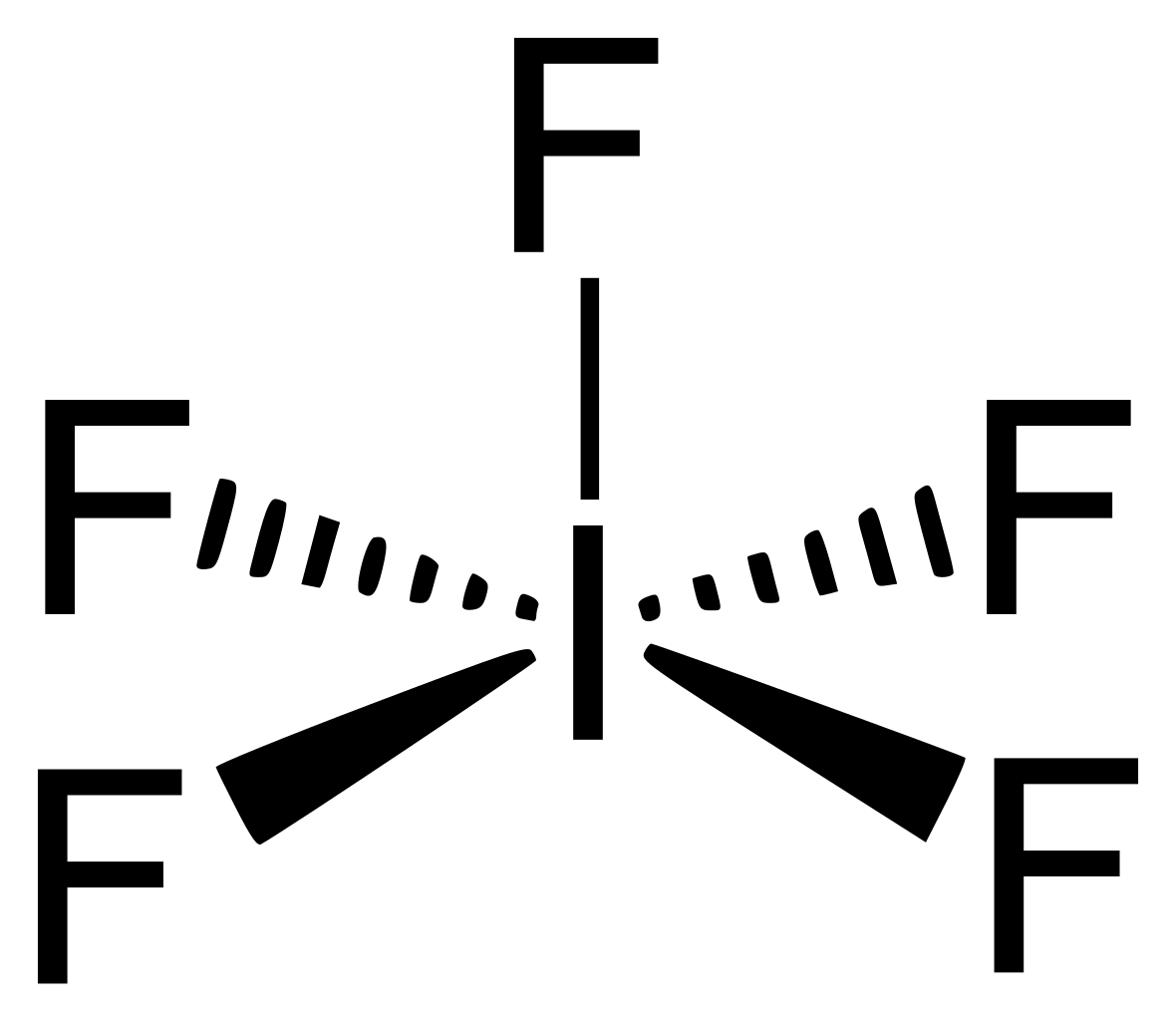
Iodine Lewis Dot Diagram

Iodine pentafluoride IF5 Molecular Geometry Hybridization
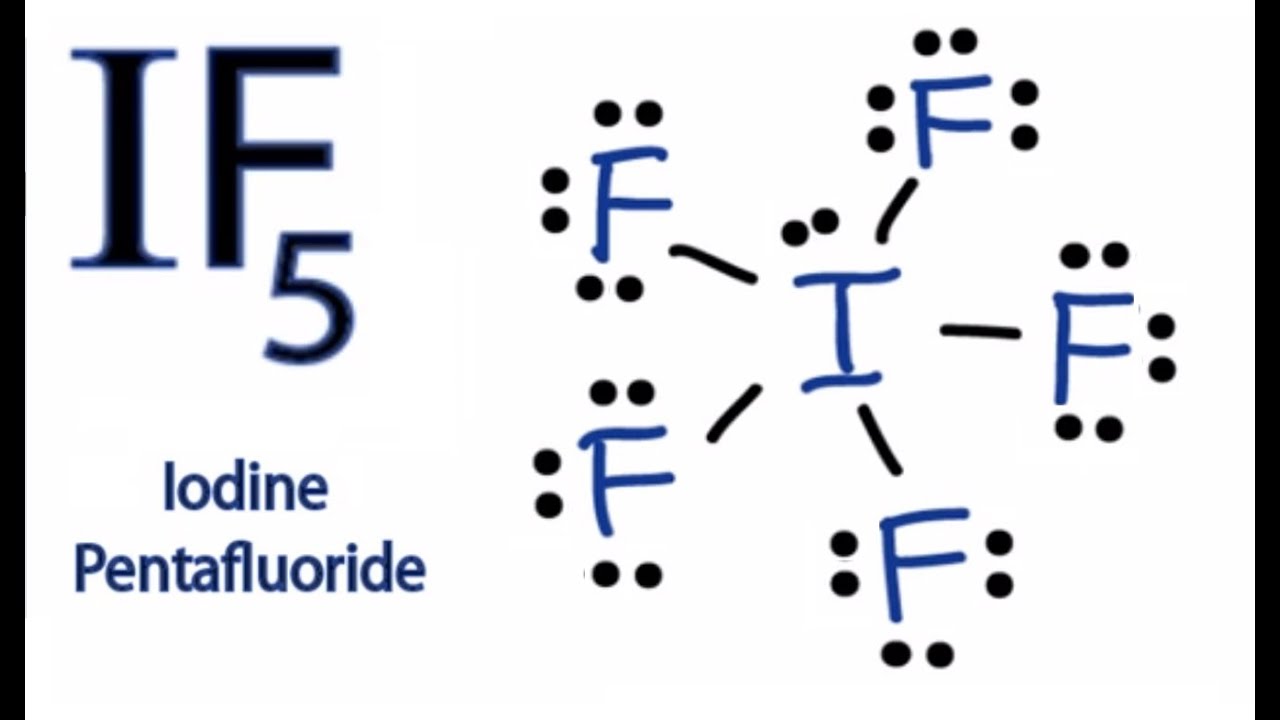
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 YouTube
We Need To Draw The Lewis Dot Structure Of Hydrogen.
There Is An Electronic Configuration Of Kr Four D 10, Five S Two, And B Five.
(B) Which Is The Stronger Bond.
This Problem Has Been Solved!
Related Post:
