Draw The Lewis Structure For The Pcl+4 Ion
Draw The Lewis Structure For The Pcl+4 Ion - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers draw the lewis structure for the (pcl4+)ion. Web drawing lewis structures for molecules with one central atom: Ocl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant. The lewis electron structure for the nh 4 + ion is as follows: Phosphorous and chlorine atoms have 5. Four pairs will be used in the chemical bonds between the p and f. This problem has been solved! Web lewis structure of pcl 4+. Web draw lewis structures for ionic compounds. Pcl 4+ has one phosphorus atom and four chlorine atoms. Web pcl4+ lewis structure contains 32 total valence electrons. Web chemistry chemistry questions and answers draw the lewis structure for the (pcl4+)ion. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web pcl4+ is a. Cl assign a formal charge to each atom in the student's lewis structure atom formal charge left cl top cl right cl bottom cl this problem has been solved! Web draw the lewis structure for the pcl+4 ion. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. For the pcl4+ structure use the. Web chemistry chemistry questions and answers draw the lewis structure for the (pcl4+)ion. First, we need to draw the lewis structures for the reactants and products. Show transcribed image text expert answer transcribed image text: Web the lewis electron structure is drawn within brackets as is customary for a molecular ion, with the overall charge indicated outside the brackets, and. Here are the steps and final. Phosphorous and chlorine atoms have 5. Web draw the lewis structure for the (pcl_(4)^(+))ion. For the pcl4+ structure use the periodic table to find the total number of valence electrons for the pcl4+ molecule. Web draw lewis structures for covalent compounds. The following procedure can be used to draw lewis structure for simple molecules. This problem has been solved! The lewis electron structure for the nh 4 + ion is as follows: Ocl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant. Shared pairs of electrons are drawn as lines between atoms, while lone. Web the lewis electron structure is drawn within brackets as is customary for a molecular ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. This problem has been solved! For very simple. Ocl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant. Web draw the lewis structure for the pcl+4 ion. Web drawing lewis structures for molecules with one central atom: A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. For the pcl4+ structure use the periodic table. This problem has been solved! The drive for eight the octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. For the pcl4+ structure use the periodic table to find the total number of valence electrons for the pcl4+ molecule. Also, there is a positive (+1) charge on the phosphorus atom. Web. This problem has been solved! In the lewis structure of pcl 4+, there are four single bonds around the phosphorus atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. The drive for eight the octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence. Phosphorous and chlorine atoms have 5. Web draw the lewis structures for each of the following ions or molecules. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web pcl4+ is a chemical formula for tetrachloro phosphanium ion. Draw the lewis structure for the (pcl4+)ion. Lone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure. The lewis electron structure for the nh 4 + ion is as follows: Two rules in chem 101a, we will focus on drawing lewis structures of molecules and polyatomic ions that have one central atom with several other atoms attached to it. Web augchem 436 subscribers 1.8k views 3 years ago i needed to draw pcl4+ so i could show how to input it into the chemdoodle chemical drawing program in owl. Web pcl4+ lewis structure contains 32 total valence electrons. Web draw lewis structures for covalent compounds. Four pairs will be used in the chemical bonds between the p and f. Show transcribed image text expert answer transcribed image text: Web lewis structure of pcl 4+. Here are the steps and final. Web drawing lewis structures for molecules with one central atom: This problem has been solved! The drive for eight the octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. First, we need to draw the lewis structures for the reactants and products. Ocl − is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
How To Draw Lewis Dot Structures For Compounds

Lewis Dot Structure Definition, Examples, and Drawing
Lewis Structure Practice Worksheet

3 Ways to Draw Lewis Dot Structures wikiHow
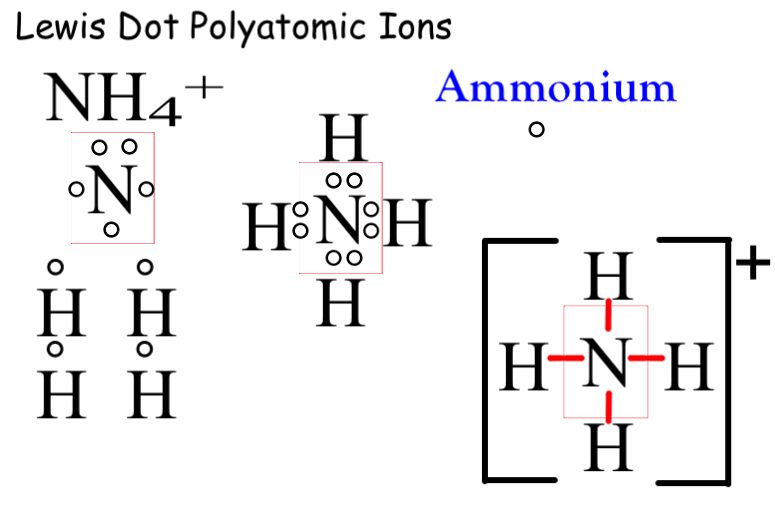
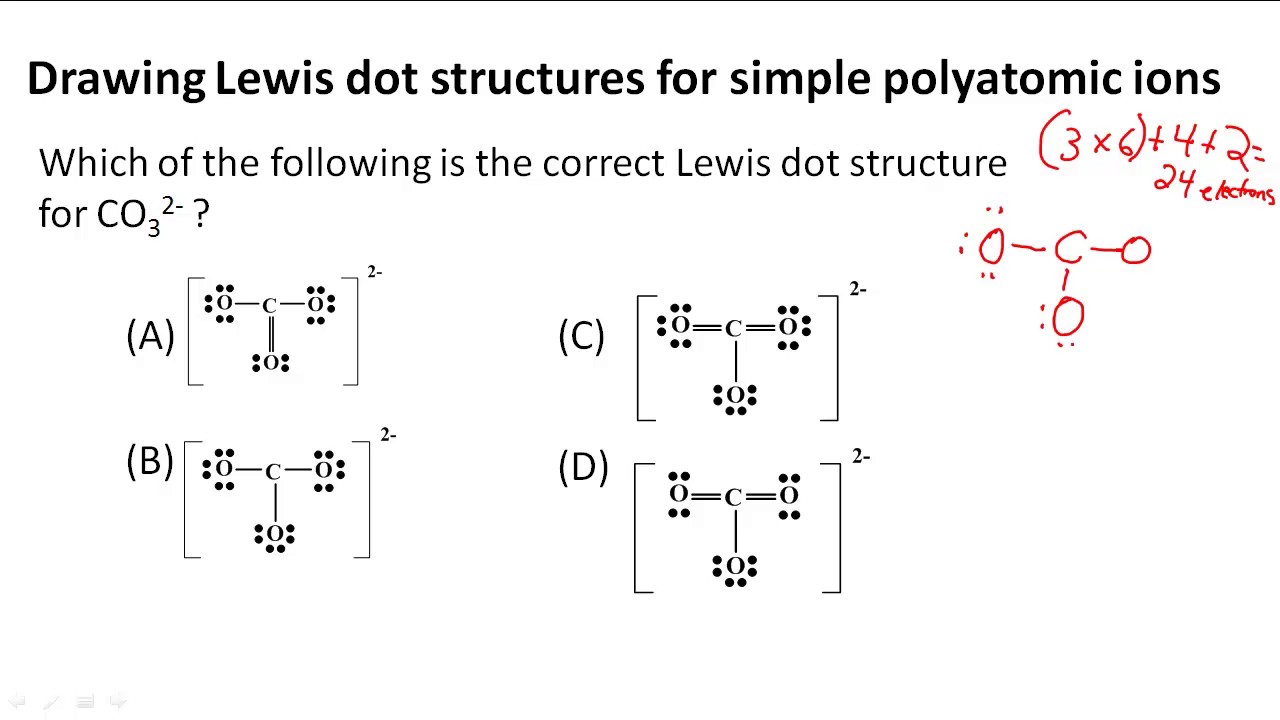
How do you draw lewis structures for polyatomic ions? Socratic

Draw the Lewis Structure for the Pcl+4 Ion
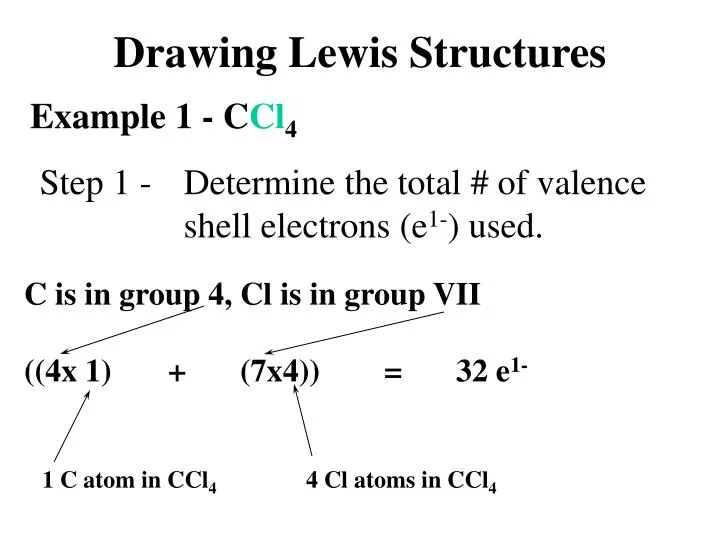
How To Draw Lewis Structures For Polyatomic Ions

How to Draw a Lewis Structure

How To Draw Lewis Structures Abilitystop

How to Draw the Lewis Structure for PCl4+ YouTube
Web Each Chlorine (At No.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw The Lewis Structure For The (Pcl4+)Ion.
For The Pcl4+ Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For The Pcl4+ Molecule.
Related Post: