Draw The Lewis Structure Of Calcium Chloride
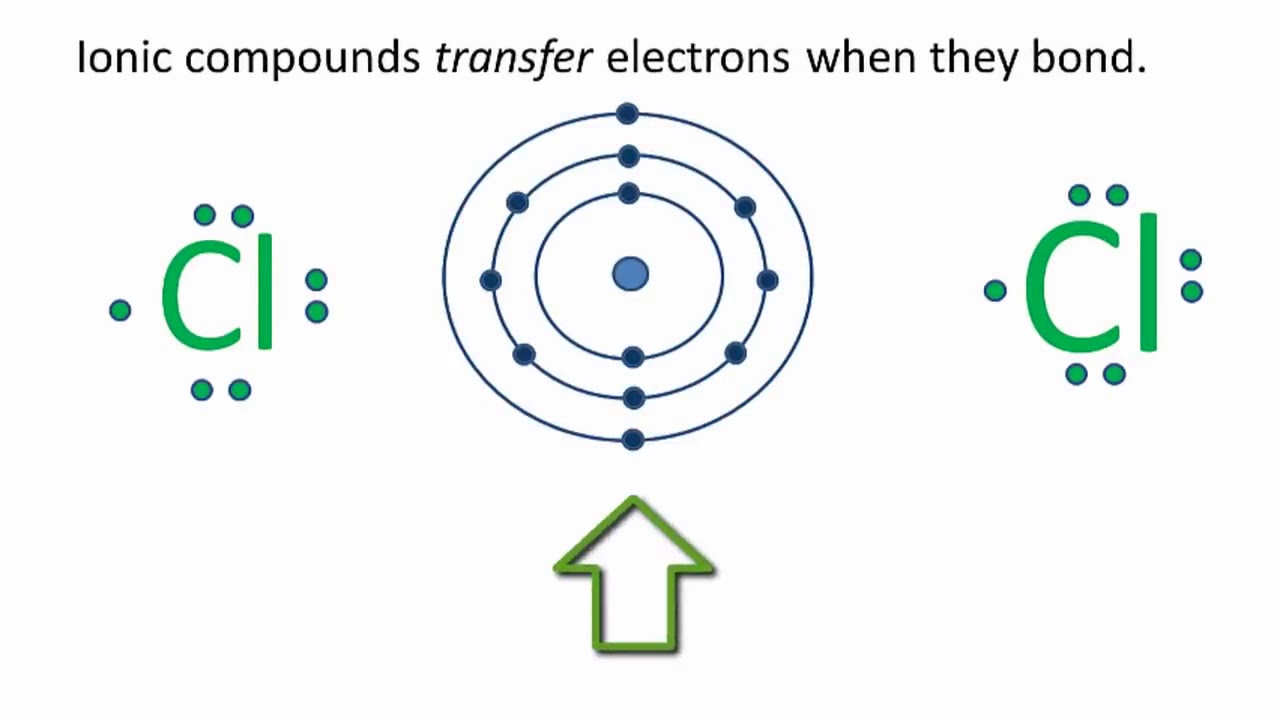
Draw The Lewis Structure Of Calcium Chloride - Web cacl2 is a chemical formula for calcium chloride as it consists of one calcium and two chlorine atoms. What kind of bond forms between a calcium atom and a chloride atom? The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Draw the lewis structure of calcium chloride. First, lets find the how. Web draw the lewis structure of calcium chloride. Web draw the lewis structure for calcium chloride? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. X this problem has been solved! Web draw the lewis dot structure for the chloride ion. Learn the definition of octet rule and lewis structure. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: First, lets find the how. Web as you can see,. Web steps to draw the lewis structure of cacl2. X this problem has been solved! Web draw the lewis dot structure for the chloride ion. How to draw the lewis dot structure for calcium chloride sevil atun 79 subscribers subscribe subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9. Draw the lewis structure of calcium chloride. X this problem has been solved! Draw the lewis structure for hcl. Web draw the lewis dot structure for the chloride ion. Calcium lewis dot structure with oxygen (cao) next, let’s consider the lewis dot structure of calcium when it forms a compound with oxygen, resulting in calcium oxide (cao). Web draw the lewis dot structure for the chloride ion. Web the configuration of calcium with 20 electrons can be written \[ca:\, [ar]4s^2 \label{3}\]. Web cacl2 lewis structure: Web how to draw cacl2 lewis structure? Calcium atom has two valence electron and each chlorine atom has seven valance electrons. As it is an ionic compound, we will follow a slightly. Oxygen has six valence electrons, while calcium has two. Web the first step is to sketch the lewis structure of the cacl2 molecule, to add valence electron around the calcium atom; Learn the definition of octet rule and lewis structure. Draw the lewis structure for hcl. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. One calcium atom loses two electrons, so it becomes +2 charge two chlorine atoms gain those. Web cacl2 is a chemical formula for calcium chloride as it consists of one calcium and two chlorine atoms. This problem has been solved! Draw the lewis structure. Web cacl2 is a chemical formula for calcium chloride as it consists of one calcium and two chlorine atoms. Web draw the lewis dot structure for the chloride ion. Web steps to draw the lewis structure of cacl2. Web drawing lewis structures for molecules with one central atom: Draw the lewis structure of calcium chloride. What kind of bond forms between a calcium atom and a chloride atom? Web how to draw cacl2 lewis structure? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Calcium chloride (cacl2 or cl2ca) is an ionic compound. Count all valence electrons available in cacl2 in the first stage. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. We are asked to draw the lewis structure for calcium chloride. Calcium chloride is an ionic compound. Web the configuration of calcium with 20 electrons can be written \[ca:\, [ar]4s^2 \label{3}\]. Count all valence electrons available in cacl2 in the first. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the first step is to sketch the lewis structure of the cacl2 molecule, to add valence electron around the calcium atom; Web the configuration of calcium with 20 electrons can be written \[ca:\, [ar]4s^2 \label{3}\]. X this problem has been solved! Draw the. Draw the lewis structure of calcium chloride. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Calcium chloride (cacl2 or cl2ca) is an ionic compound. Calcium chloride is an ionic compound. The number of valence electrons in calcium chloride molecule has to be counted first. Web cacl2 is a chemical formula for calcium chloride as it consists of one calcium and two chlorine atoms. What kind of bond forms between a calcium atom and a chloride atom? One calcium atom loses two electrons, so it becomes +2 charge two chlorine atoms gain those. This is required by the law of conservation of matter as well. Web 21k views 3 years ago lewis structures. Calcium belongs to 2nd group and the valence. We are asked to draw the lewis structure for calcium chloride. Web as you can see, the lewis dot structure for a calcium ion consists of two dots around the symbol ca. Learn the definition of octet rule and lewis structure. First, lets find the how. The cacl2 molecule has a linear shape because it contains two.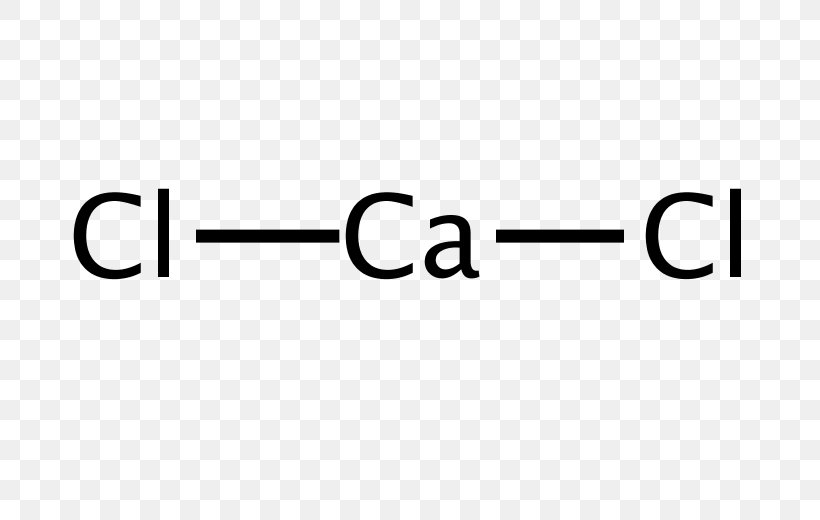
Calcium Chloride Lewis Structure Chemistry, PNG, 696x520px, Calcium
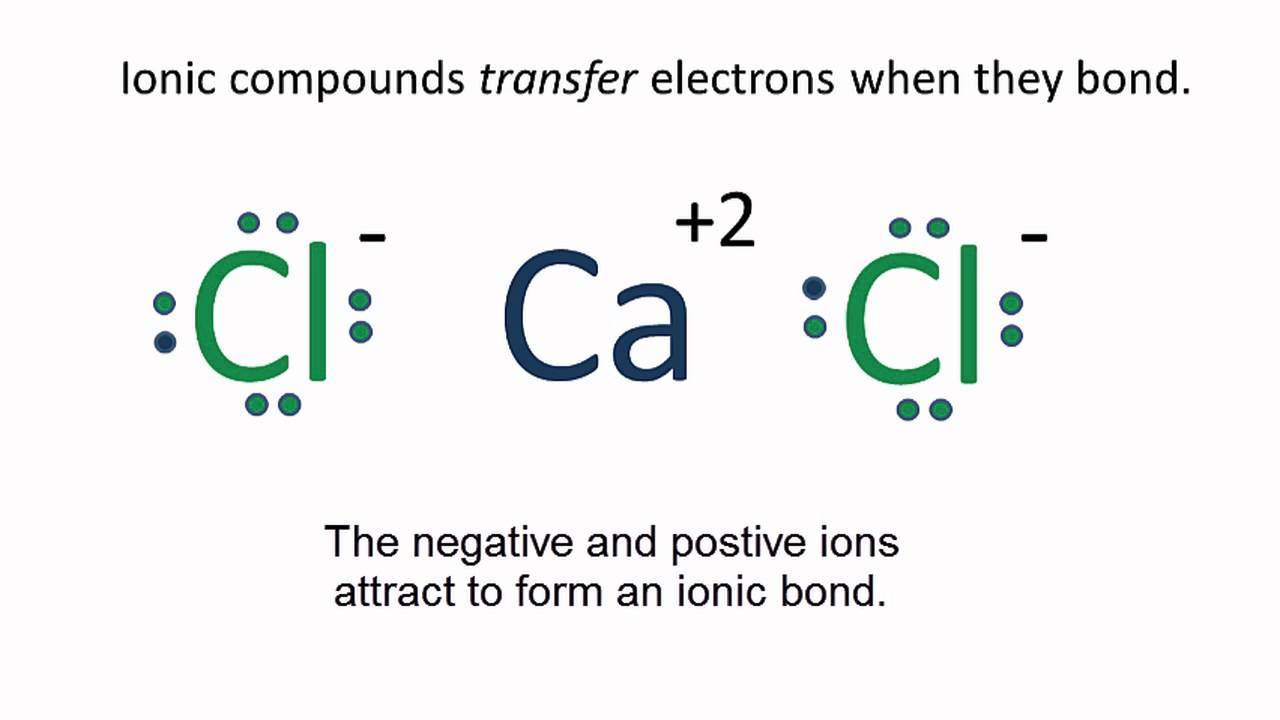
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium
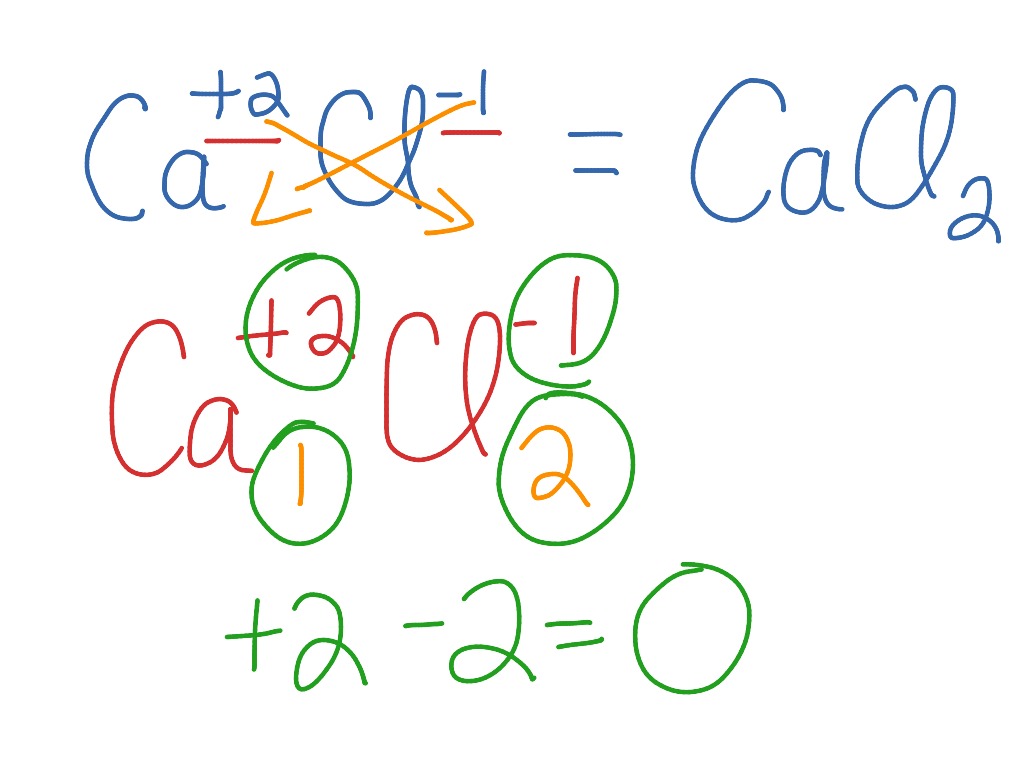
Formula for Calcium chloride Science, Chemistry ShowMe

Draw the Lewis Structure of CaCl2 (calcium chloride) YouTube
Cacl2 Lewis Structure How To Draw The Lewis Dot Structure
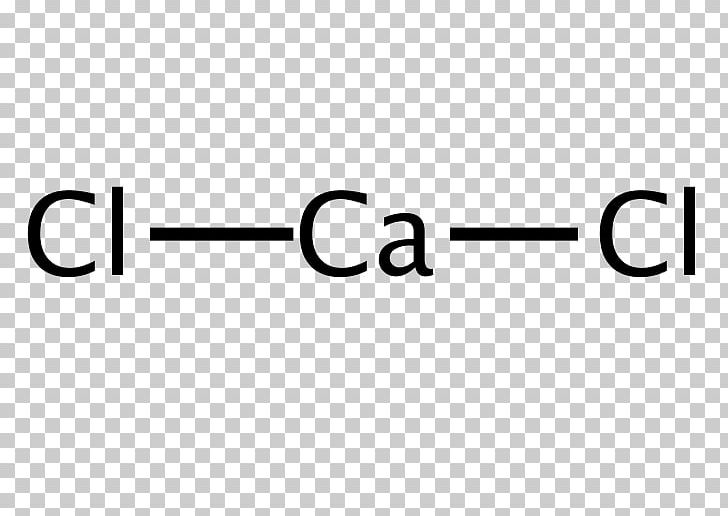
Calcium Chloride Lewis Structure Chemistry PNG, Clipart, Angle, Area
Calcium Chloride Lewis Dot Structure
ShowMe Lewis electron dot structure for calcium chloride
![[Expert Verified] write electron dot diagram for chlorine and Calcium](https://hi-static.z-dn.net/files/d37/af53c843c5f23744299a8113155f3696.jpg)
[Expert Verified] write electron dot diagram for chlorine and Calcium
Calcium Atom Has Two Valence Electron And Each Chlorine Atom Has Seven Valance Electrons.
Web Lewis Structures (Also Known As Lewis Dot Diagrams, Electron Dot Diagrams,Lewis Dot Formula Lewis Dot Structures, And Electron Dot Structures) Are Diagrams That Show The Bonding Between Atoms Of A Molecule And The Lone Pairs Of.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Count All Valence Electrons Available In Cacl2 In The First Stage.
Related Post: