Draw The Lewis Structure Of Ch3Br
Draw The Lewis Structure Of Ch3Br - The first step is to sketch the molecular geometry of the ch3br molecule, to calculate the lone pairs of the electron in the central carbon, terminal bromine, and terminal hydrogen atom; For the ch3br structure use the periodic table to find the total number of valence electrons for the ch3br. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The bond length between the carbon and bromine. Web how to draw ch3br lewis structure? Draw the molecule by placing atoms on the grid and connecting them with bonds. Determine the central atom in the molecule. Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. The bond angle around each carbon atom is 109.5°.4. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. The central atom is carbon, which is bordered on four terminals with one bromine atom, three. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central. For the ch3br molecule (c is the central atom), draw the correct lewis structure and select all polar covalent bonds. We will learn how to draw lewis structure of ch 3 br step by step in this tutorial. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Count the total number of valence. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. In order to draw the lewis. This widget gets the lewis structure of chemical compounds. Web drawing lewis structures for molecules with one central atom: The second step is to calculate the ch3br hybridization, and the third step is to give. You can intuitively draw any lewis structure on your computer or mobile device. Carbon atom is the center atom and bromine atom has 3 lone pairs. Web see the big list of lewis structures. In this post, we discussed the method to construct the ch3br lewis structure. Web the lewis structure for ch3br is shown below: • we will begin by calculating the total number of valence electrons in this molecule. We will learn how to draw lewis structure of ch 3 br step by step in this tutorial. The type of hybridization around the br atom is sp3.3. Draw the lewis structure of ch3br draw the lewis structure of ch3br here’s the best way to. Web in this section, we will draw the lewis structure of the ch 3 br molecule step by step: • we will begin by calculating the total number of valence electrons in this molecule. Web this is the total number of electrons that must be used in the lewis structure. So the total number of valence electrons in ch3br is. Carbon atom is the center atom and bromine atom has 3 lone pairs. You can intuitively draw any lewis structure on your computer or mobile device. While selecting the center atom, always put the least. The type of hybridization around each carbon atom is sp3.2. In order to draw the lewis. In the case of ch3br, carbon being a group. In order to draw the lewis. Web to draw the ch3br lewis structure, follow these steps: Ch 3 br lewis structure Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. For the ch3br structure use the periodic table to find the total number of valence electrons. Web ch3br lewis structure has a carbon atom (c) at the center which is surrounded by three hydrogen atoms (h) and one bromine atom (br). There are 3 lone pairs on the bromine atom (br). While selecting the center atom, always put the least.. The bond length between the carbon and bromine. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web in this section, we will draw the lewis structure of the ch 3 br molecule step by step: This widget gets the lewis structure of chemical compounds. The central atom is carbon,. Hydrogen always goes on the outside, and since carbon is less electronegative than bromine, we'll put the carbon in the center and the bromine on top. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. It has only one carbon atom. While selecting the center atom, always put the least. Web this is the total number of electrons that must be used in the lewis structure. Carbon atom is the center atom and bromine atom has 3 lone pairs. Web check me out: The central atom is carbon, which is bordered on four terminals with one bromine atom, three. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. This widget gets the lewis structure of chemical compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The type of hybridization around the br atom is sp3.3. So the total number of valence electrons in ch3br is (4 + 3*1 + 7) = 14. Include all lone pairs of electrons. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When the question is first opened, a box with a.
CH3Br Lewis Structure, Geometry, Hybridization, and Polarity

How to draw CH3Br Lewis Structure? Science Education and Tutorials

CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
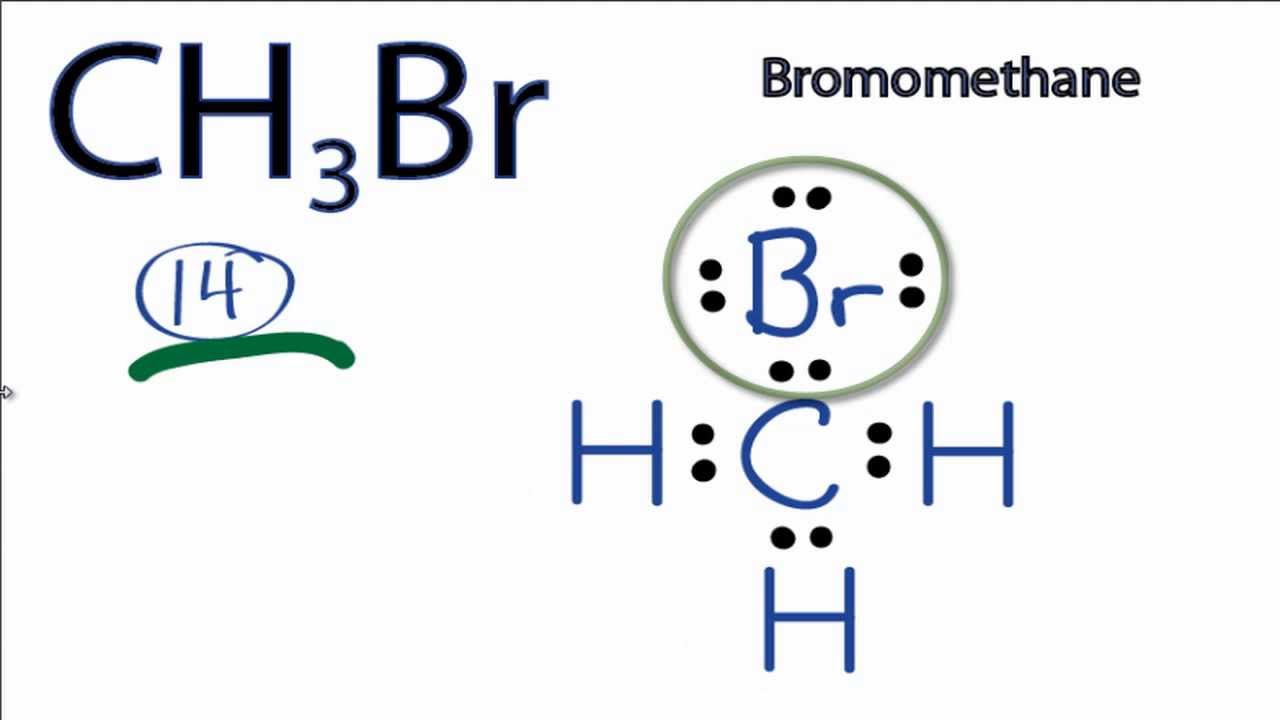
CH3Br Lewis Structure How to Draw the Lewis Structure for CH3Br

How to draw CH3Br Lewis Structure? Science Education and Tutorials

CH3Br (Bromomethane) Molecular Geometry, Bond Angles YouTube

CH3Br Lewis Structure (Bromomethane) YouTube

CHBr3 Molecular Geometry Science Education and Tutorials
[Solved] Draw the Lewis structure of bromoform (CH3Br). a. What is the

draw the lewis structure of ch3br sectionalviewsengineeringdrawing
Web By Using The Following Steps, You Can Easily Draw The Lewis Structure Of Ch 3 Br.
Draw A Skeleton Structure Of The Molecule Or Ion, Arranging The Atoms Around A Central Atom And Connecting Each Atom To The Central Atom With A Single (One Electron Pair) Bond.
For The Ch3Br Molecule (C Is The Central Atom), Draw The Correct Lewis Structure And Select All Polar Covalent Bonds.
#1 Draw Skeleton #2 Show Chemical Bond #3 Mark Lone Pairs #4 Calculate Formal Charge And Check Stability (If Octet Is Already Completed On Central Atom) Let’s One By One Discuss Each Step In Detail.
Related Post: