Draw The Lewis Structure Of Hcn. Include Lone Pairs.
Draw The Lewis Structure Of Hcn. Include Lone Pairs. - Web draw the lewis dot structure for the molecule. Add these electrons to give every atom an octet you nave to put a triple bond between c and n. A triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Web hcn lewis structure: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the carbon atom has (or shares) 3 electrons from the triple bond, and a lone pair of electrons, which it owns. Atoms are arranged in a circle around the nitrogen atom, which is in the center of the structure. The nitrogen atom has 1 lone pair. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Web science chemistry chemistry questions and answers draw the lewis structure for the hcn molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This is the complete lewis structure of co 2. The single bond is active by default. Web moving one lone pair from each terminal o atom, the following structure is obtained. The lone pairs are shown as small circles, and the hydrogen atoms are. Web how to draw the lewis structure of hcn? Web the lewis structure of hcn shows a triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. However, there are two possible resonance structures that can be drawn for hcn: Draw a skeleton structure put the least electronegative atom c in the. Then, use single bonds to link carbon, hydrogen, and nitrogen. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. Web how to draw the lewis structure of hcn? This is the complete lewis structure of co 2. In this method, we find the bonds and lone pairs for the. For the hcn lewis structure, calculate the total number of valence electrons for the hcn. However, there are two possible resonance structures that can be drawn for hcn: Write a lewis structure for each of the following polyatomic ions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Let’s draw and understand this. Draw the lewis structure for. The nitrogen atom is surrounded by four hydrogen atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the lewis structure of hcn shows a triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Step method to draw. Step by step solved in 2 steps with 1 images see solution You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web hcn lewis structure: Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a. Web draw the lewis dot structure for the molecule. The carbon atom (c) is at the center and it is surrounded by hydrogen (h) and nitrogen atom (n). Draw the lewis structure for. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. You'll get a detailed solution from. To draw the lewis structure of hcn, put carbon (c) in the middle, hydrogen (h) on one side, and nitrogen (n) on the other. The nitrogen atom has 1 lone pair. Web chemistry chemistry questions and answers draw the lewis structure for hcn. With 2 inner core electrons, this makes 7 electrons with which it is associated. Write a lewis. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Hcn lewis structure once you get the total number of valence electrons, you can make a lewis dot structure of hcn. Step by step solved in 2 steps with 1 images see solution Step method to draw the lewis structure of. Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen. The carbon atom (c) is at the center and it is surrounded by hydrogen (h) and nitrogen atom (n). The single bond is active by default. Count the valence electrons you can use h + c + n =1 + 4 + 5 =. Put one electron pair in each bond4. This is the complete lewis structure of co 2. Put least electronegative atom in centre3. However, there are two possible resonance structures that can be drawn for hcn: Draw the lewis structure for hcn. Draw the lewis structure for. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure Draw the lewis structure of hcn.hcn. For those ions that exhibit resonance, draw the various possible resonance forms. Draw the lewis structure of. To draw the lewis structure of hcn, put carbon (c) in the middle, hydrogen (h) on one side, and nitrogen (n) on the other. Web science chemistry chemistry questions and answers draw the lewis structure for the hcn molecule. Add these electrons to give every atom an octet you nave to put a triple bond between c and n. Web moving one lone pair from each terminal o atom, the following structure is obtained. Since, the atomic number of carbon is 6, the carbon atom is formally negatively charged. Fill out the octet of nitrogen with a lone pair.
Lewis Diagram For Hcn
[Solved] Draw the Lewis structure of HCN. Include lone pairs. Select
[Solved] Draw the Lewis structure of HCN. Include lone pairs. Select

Hcn Lewis Structure Bonds Draw Easy
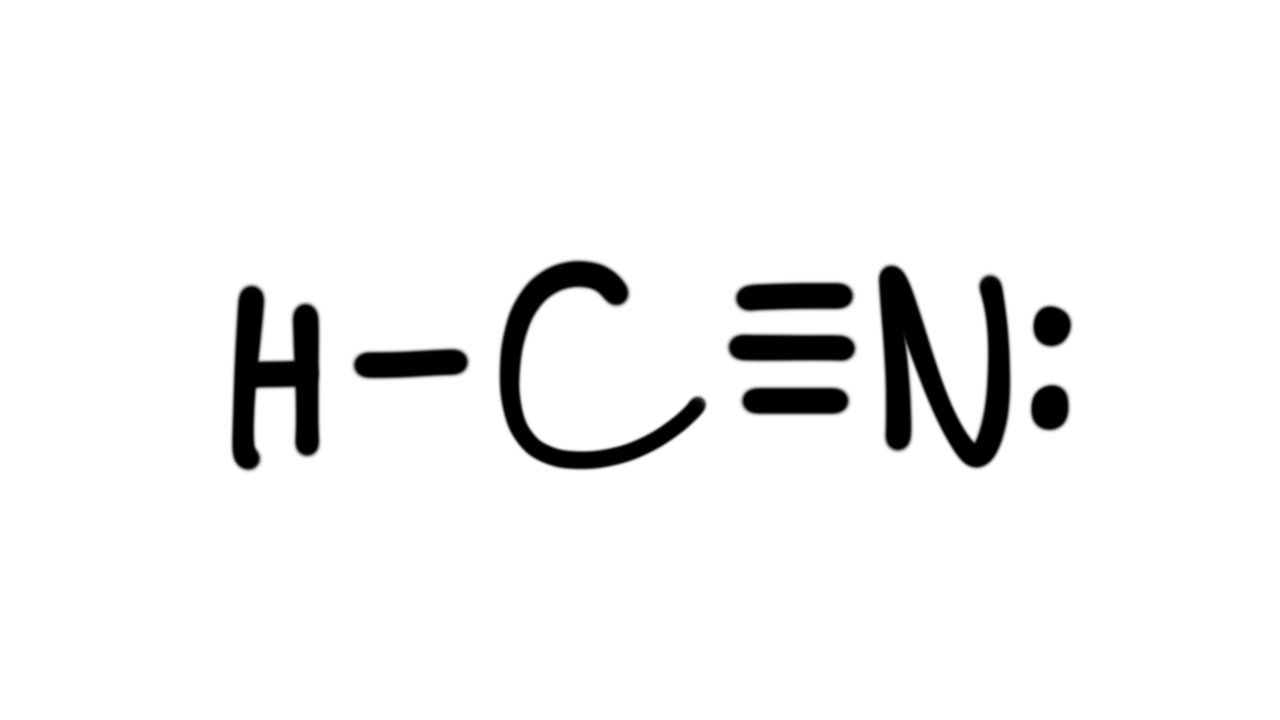
Lewis Diagram For Hcn
[Solved] draw the lewis structure of CO2. include lone pairs Draw the
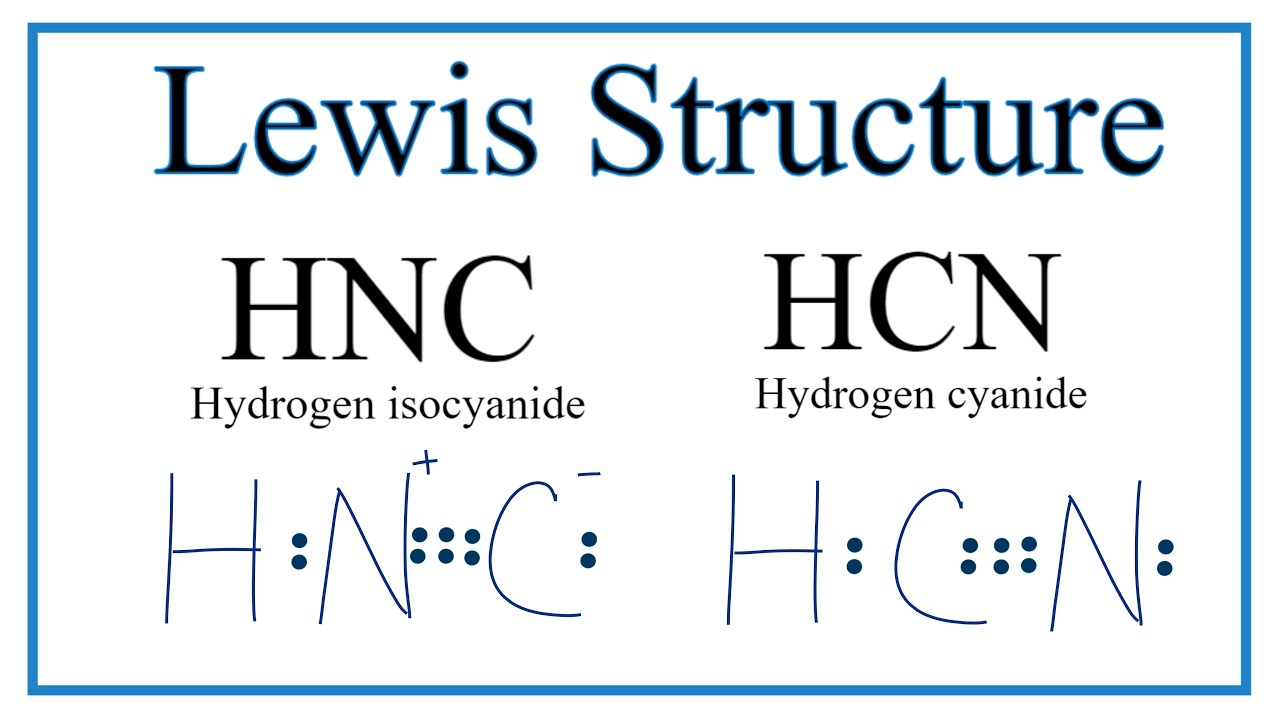
Hcn Lewis Structure Bonds Draw Easy

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
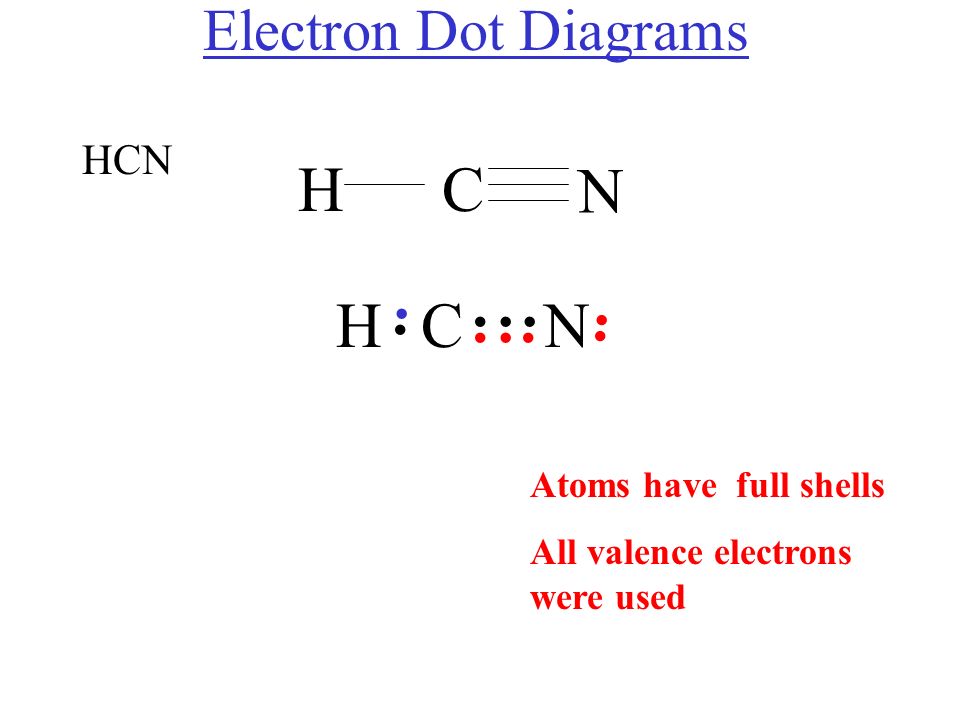
Lewis Diagram For Hcn
Write The Lewis Structure For The Molecule.
Draw The Molecule On The Canvas By Choosing Buttons From The Tools (For Bonds), Atoms, And Advanced Template Toolbars.
Web First Of All, To Remind You Lewis’s Structure Is A Pictorial Representation Of Different Bonds And Lone Pair Of Electrons Between Two Or More Atoms Of A Compound.
This Problem Has Been Solved!
Related Post: