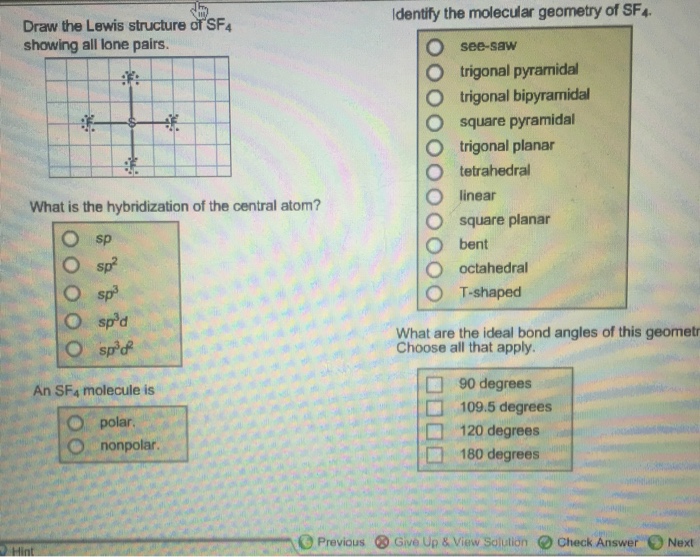
Draw The Lewis Structure Of Sf4 Showing All Lone Pairs
Draw The Lewis Structure Of Sf4 Showing All Lone Pairs - Identify the molecular geometry of sfa. Lewis structures show all of the valence electrons in an atom or molecule. Next, select draw rings to generate the ring structure around the lewis structure.3. In total, we have 6 + 4 (7) = 34 valence electrons. The component in the question is declared a mitten. The lewis structure of sf2 needs to be written down in this question. Identify the molecular geometry of sf4. Web draw the lewis structure of sf2, showing all lone pairs. The central atom in this compound is sulfur. Sf4 lewis structure is made up of one sulfur (s), and four fluorine (f) atoms. What is the hybridization of the central atom? The lewis structure is what we will start with. Finally, erase any unnecessary portions of the ring structure. The lewis structure of sf2 needs to be written down in this question. Web the lewis structure of sf4 consists of one sulfur (s) atom and four fluorine (f) atoms. The lewis structure is what we will start with. Draw the lewis structure of nh3 and determine the electron pair geometry around the central atom. We don’t have your requested question, but here is a suggested video that might help. Web draw the lewis structure of sf2, showing all lone pairs. Identify the molecular geometry of sfa. Draw the lewis structure of sf, showing all lone pairs. The lewis structure of sf4 contains 13 lone pairs and 4 bonding pairs. Lewis structures show all of the valence electrons in an atom or molecule. Next, select draw rings to generate the ring structure around the lewis structure.3. Hello students, let's start with the question. Contents sf4 molecular geometry sf4 lewis structure is sf4 polar? What is the hybridization of the central atom? Note that in the lewis structure, the lone pairs are represented by dots and the bonding pairs are simply,. Sp sp2 sp3 sp3d sp3d2 an sf4 molecule is polar. #4 calculate formal charge and check stability (if octet is already completed on. Is an sf4 molecule polar or nonpolar? What is the hybridization of the central atom? Identify the molecular geometry of sfa. The component in the question is declared a mitten. Web draw the lewis structure of sf4, showing all lone pairs. Draw the lewis structure of sf4 showing all lone pairs. Sf4 lewis structure is made up of one sulfur (s), and four fluorine (f) atoms. The lewis structure of sf4 contains 13 lone pairs and 4 bonding pairs. Draw the lewis structure of sf4 showing all lone pairs. What is the hybridization of the central atom? In total, we have 6 + 4 (7) = 34 valence electrons. Web a lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. Identify the molecular geometry of sf4. Sp2 sp3d sp3d2 sp sp3 You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure of nh3 and determine the electron pair geometry around the central atom. The central atom in this compound is sulfur. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss. Web draw the lewis structure of sf4 showing all lone pairs? Draw the lewis structure of sf4 showing all lone pairs. Sf4 lewis structure is made up of one sulfur (s), and four fluorine (f) atoms. Web this problem has been solved! Sulfur is in group 16 of the periodic table, so it has 6 valence electrons. Draw the lewis structure of sf, showing all lone pairs. Web draw the lewis structure of sf2, showing all lone pairs. Web a lewis structure is a way to show how atoms share electrons when they form a molecule. The lewis structure is what we will start with. Draw the lewis structure of sf4 showing all lone pairs. Sp sp2 sp3 sp3d sp3d2 an sf4 molecule is polar. The lewis structure of sf2 needs to be written down in this question. An sf_4 molecule is identify the molecular geometry of sf_4. For the sf4 structure use the periodic table to find the total number of valence electrons for.more. This sulfur has 6 valence electrons and the fluorine has 7. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers draw the lewis structure of sf4 showing all lone pairs. The central atom in this compound is sulfur. Identify the molecular geometry of sf4. T‑shaped square pyramidal bent linear trigonal pyramidal octahedral tetrahedral trigonal planar trigonal bipyramidal see‑saw square planar what is the hybridization of the central atom? Web this problem has been solved! Web to draw the structure of sf4 we will need to know the total number of valence electrons that exist in sf4. Draw the lewis structure of nh3 and determine the electron pair geometry around the central atom. The valence electrons are the electrons in the outermost shell. Web start by drawing the lewis structure of sf4.2. The sf2's total valence electrons are equal to 6 plus 2 and
How to draw SF4 Lewis Structure? Science Education and Tutorials
Solved Draw the Lewis structure of SF_4 showing all lone
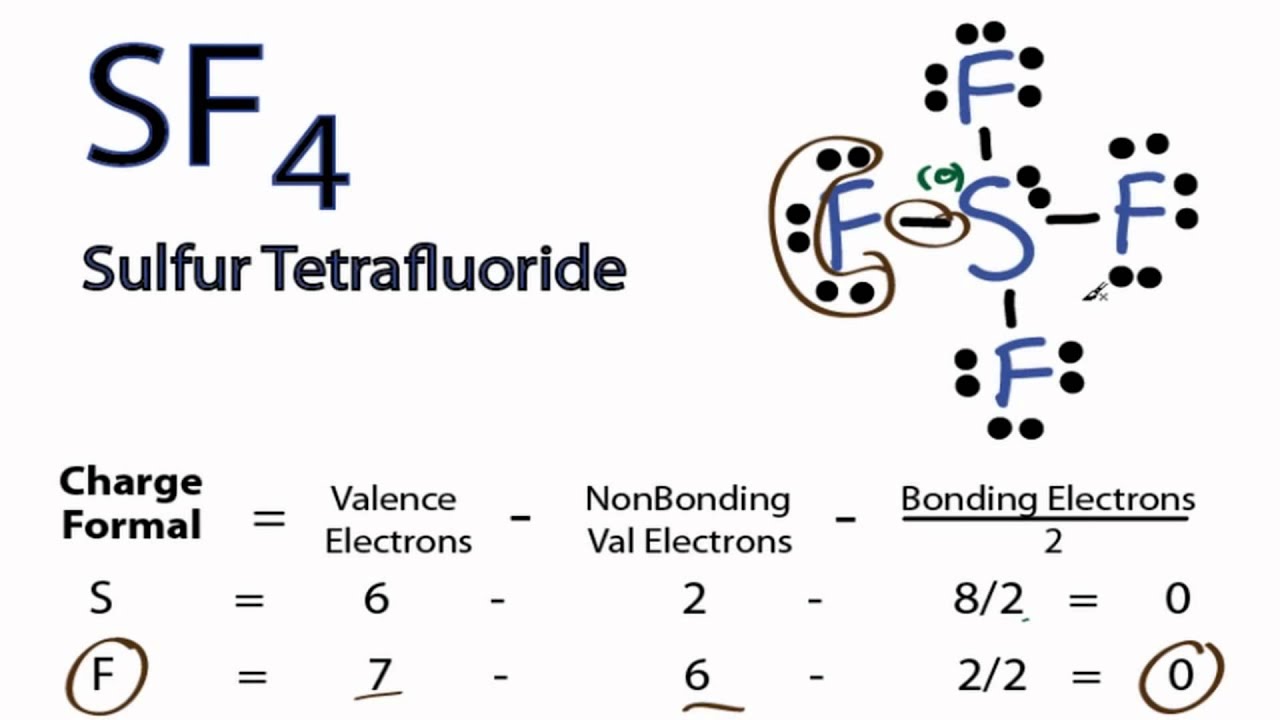
SF4 Molecular YouTube
[Solved] Draw the Lewis structure of SF 4 showing all lone pairs
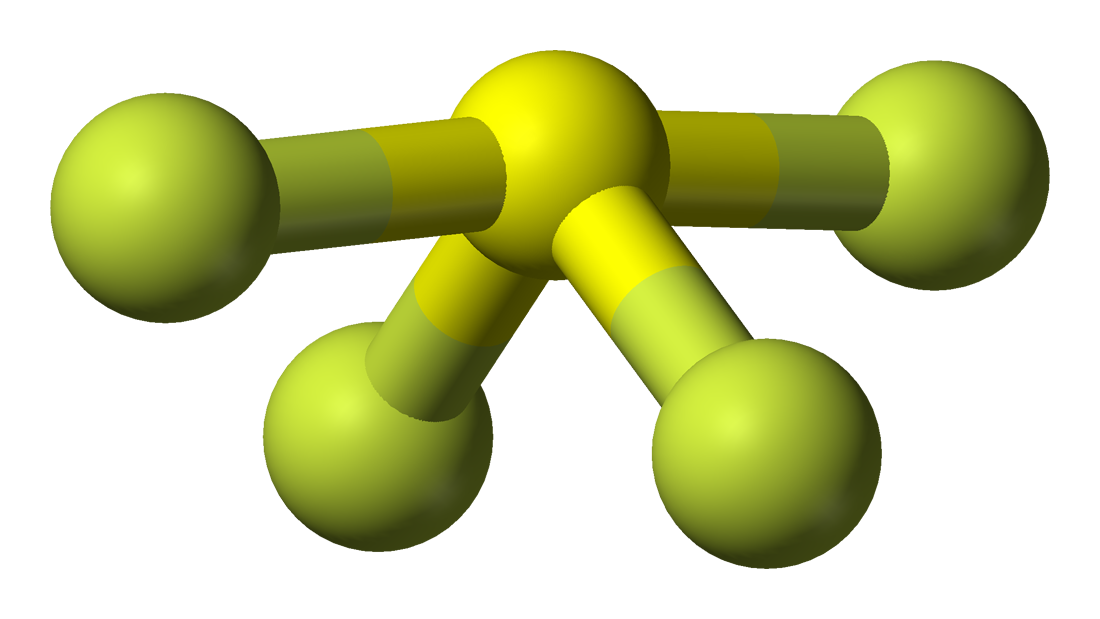
Geometría molecular del SF4, estructura de Lewis, ángulos de enlace y

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
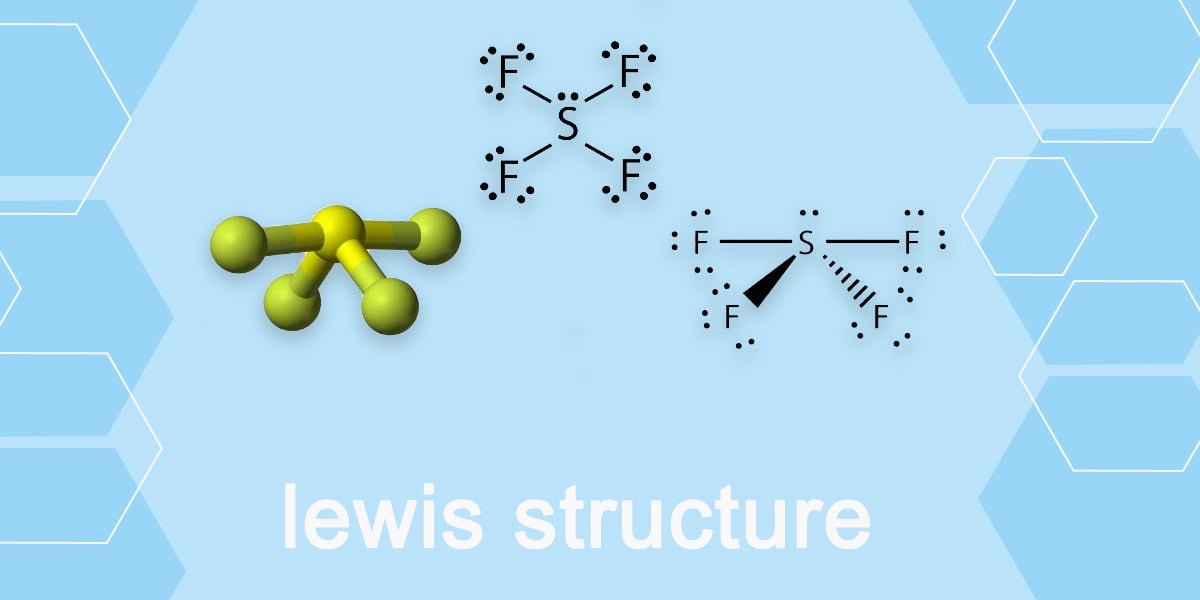
How to draw Sf4 Lewis Structure? Beginners Guide
Individually Speaking, Valence Electrons Of Sulfur = 6 Valence Electrons Of Fluorine = 7
The Component In The Question Is Declared A Mitten.
Fluorine Is In Group 17, So Each Fluorine Atom Contributes 7 Valence Electrons.
Place All Remaining Electrons On The Central Atom.
Related Post: