Draw The Lewis Structure Of Xef4 . Include Lone Pairs.
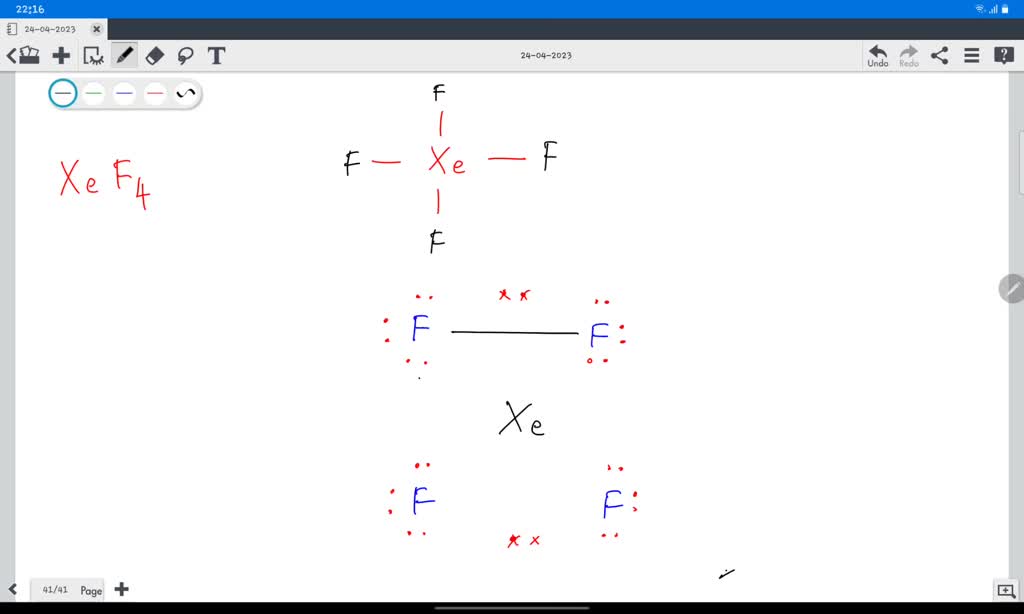
Draw The Lewis Structure Of Xef4 . Include Lone Pairs. - There are three lone pairs on each fluorine atom, and two lone pairs on the xenon atom. Therefore, the vsepr theory states that there must be minimum repulsion between the electrons. Today we are going to look at the lewis structure of xef4 ( xenon tetrafluoride ) although xenon is a noble gas it reacts with four fluorine atoms to attain a stable structure. It is a type of noble gas having the chemical equation of. Remember that lewis structures primarily show the bonding and valence electron distribution in molecules, and Xeof4 is a compound composed of xenon (xe), oxygen (o), and fluorine (f) atoms. Web the xef4 or xenon tetrafluoride is a chemical compound made of xenon and fluoride atoms. Xenon (xe) sits in the middle, and it's connected to four fluorine atoms (f) with single bonds. Web how to draw the lewis dot structure for xef4: The central xenon atom a. Web each atom of fluorine will complete its octet by adding 3 lone pairs of electrons. Has an incomplete octet c. For the central xenon atom: Today we are going to look at the lewis structure of xef4 ( xenon tetrafluoride ) although xenon is a noble gas it reacts with four fluorine atoms to attain a stable structure. Web. Therefore, the vsepr theory states that there must be minimum repulsion between the electrons. Web each atom of fluorine will complete its octet by adding 3 lone pairs of electrons. Has an expanded octet draw a lewis structure for no2. Web draw a lewis structure for xef4 and answer the following questions based on your drawing. Xeof4 is a compound. Obeys the octet rule b. Has an incomplete octet c. Xenon (xe) has two lone pairs, and each fluorine atom (f) has three lone pairs. Include all lone pairs of electrons. This problem has been solved! The first step is to sketch the lewis structure of the xef4 molecule, to add valence electrons around the xenon atom; The lewis structure helps us understand the bonding and electron distribution in the molecule. The xef4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form. Web the xef4 or xenon tetrafluoride. It is a type of noble gas having the chemical equation of. Web how to draw the lewis dot structure for xef4: This problem has been solved! Include all lone pairs of electrons. #1 draw a rough skeleton structure first, determine the total number of valence. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Web steps here’s how you can easily draw the xef 4 lewis structure step by step: Web chemistry chemistry questions and answers xenon can be the central atom of a molecule by expanding beyond an octet of electrons. This problem has been solved! This problem has. Identify the number of bonds and lone pairs in the structure. So, if you are ready to go with these 5 simple steps, then let’s dive right into it! Has an incomplete octet c. The lewis structure helps us understand the bonding and electron distribution in the molecule. There are three lone pairs on each fluorine atom, and two lone. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Web xef4 lewis structure has xenon atom (xe) at the center which is surrounded by four fluorine atoms (f). The lewis structure helps us understand the bonding and electron distribution in the molecule. Xenon (xe) sits in the middle, and it's connected to four fluorine. The xef4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form. For, xef 4, total pairs of electrons are 18 (=36/2) in their valence shells. Identify the number of bonds and lone pairs in the structure. Xenon (xe) sits in the middle, and it's connected to four fluorine atoms (f) with single. Xeof4 is a compound composed of xenon (xe), oxygen (o), and fluorine (f) atoms. The second step is to add valence electrons to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the xef4 lewis structure. There are three lone pairs on each fluorine atom, and two lone pairs on the xenon. Web to determine the number of lone pairs and bonding pairs of electrons for xef4 we first need to draw as valid lewis structure. Identify the number of bonds and lone pairs in the structure. 4 bonds and 6 lone pairs 4 bonds and 14 lone pairs 2 bonds and 8 lone pairs the correct answer is not shown 4 bonds and 7 lone this problem has been solved! Obeys the octet rule b. Has an incomplete octet c. Web xef4 lewis structure has xenon atom (xe) at the center which is surrounded by four fluorine atoms (f). (general chemistry) this problem has been solved! Web in xef4, xenon has two lone pairs of electrons and four bonding pairs. It is a type of noble gas having the chemical equation of. Draw the lewis structure of xef4. Web chemistry chemistry questions and answers draw the lewis structure for xef4 in the window below and then answer the questions that follow. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Xenon (xe) has two lone pairs, and each fluorine atom (f) has three lone pairs. Include all lone pairs of electrons. Web chemistry chemistry questions and answers xenon can be the central atom of a molecule by expanding beyond an octet of electrons. Remember that lewis structures primarily show the bonding and valence electron distribution in molecules, and
XeOF4 Lewis Structure, Geometry, Hybridization, and Polarity

Draw The Lewis Structure Of Xef4 Include Lone Pairs Fotodtp

XeOF4 Lewis Structure How to Draw the Lewis Structure for XeOF4 YouTube

XeF4 Lewis structure, Molecular geometry, Bond angle, Shape

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube
SOLVED How many lone pairs of electrons are there in the Lewis

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram

XeF4 Lewis Structure How to Draw the Lewis Structure for XeF4 YouTube

Step2 Lewis Structure of XeF4 for counting valence electrons around

So far, we’ve used eight of the XeF4 Lewis structure’s total 8
The Lewis Structure Of Xef4 Contains Four Single Bonds, With Xenon In The Center, And Four Fluorines On Either Side.
Has An Expanded Octet Draw A Lewis Structure For No2.
Web The Lewis Dot Structure Shows The Unpaired Electrons Or The Lone Pairs In The End.
Web Chemistry Chemistry Questions And Answers Write The Lewis Structure For Xef4.
Related Post: