Draw The Main Lewis Structure Of Nof
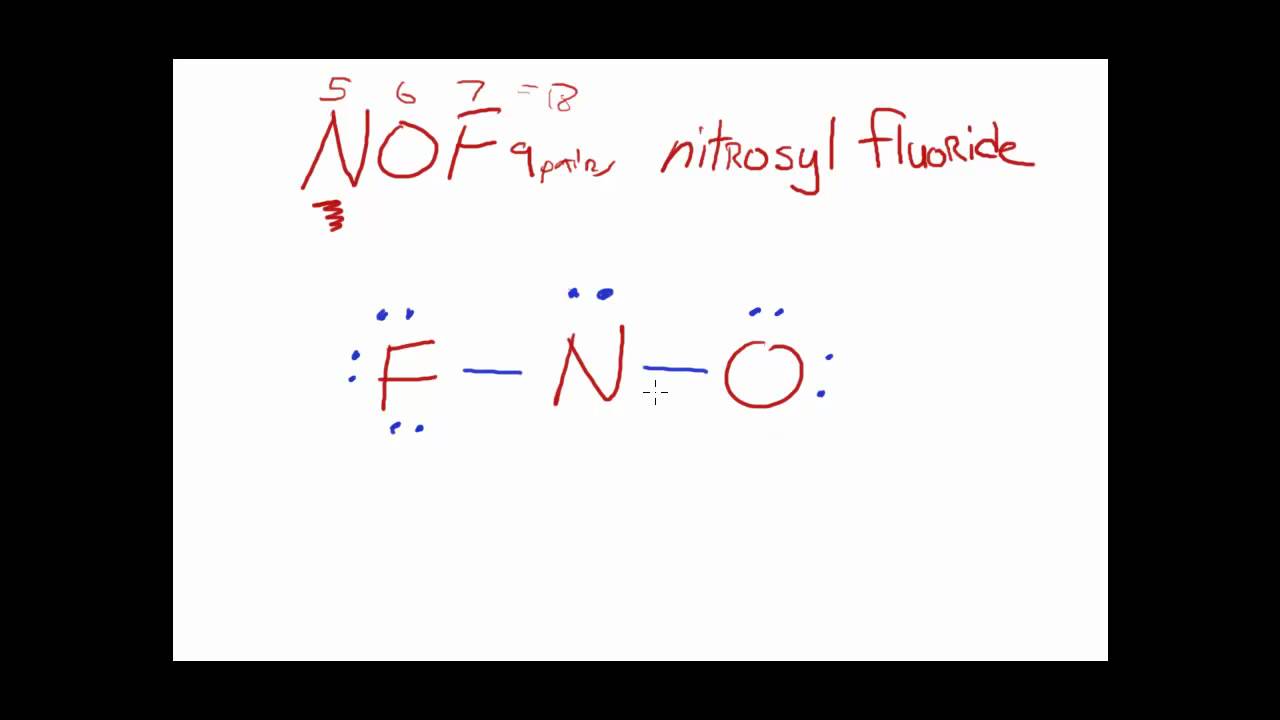
Draw The Main Lewis Structure Of Nof - Well, you’re in luck because in this article, we will show you exactly how to do it step by step. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. In order to draw the lewis. Now counting the number of valence electrons in the molecule: Web introduction draw the lewis structure of nof (nitrosyl fluoride) chemistnate 248k subscribers subscribe 4.5k views 2 years ago nof is actually onf, since nitrogen has a higher bonding. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and molecular geometries. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure Web in this task, we need to draw the main lewis structure of n o f \ce{nof} nof using dot notation for nonbonding electrons and bonds for bonding electrons. O has 1 lone pair, and f has 3 lone pairs. Web jamesripley report flag outlined final answer: Web chemistry chemistry questions and answers part b draw the main lewis structure of nof. Web 6 steps to draw the lewis structure of nof step #1: Web science chemistry chemistry questions and answers draw the lewis structure for nof nitrogen is the central atom draw the molecule by placing atoms on the grid and connecting them with bonds. Draw. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure Web drawing lewis structures for molecules with one. Thereafter, the valence electrons of all the three atoms. Web in this task, we need to draw the main lewis structure of n o f \ce{nof} nof using dot notation for nonbonding electrons and bonds for bonding electrons. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms,. Web in this task, we need to draw the main lewis structure of n o f \ce{nof} nof using dot notation for nonbonding electrons and bonds for bonding electrons. View available hint (s) ννου ch on spf brax more this problem has been solved! For the nof structure use the periodic table to find the total number of valence electrons. Web definition and purpose the main purpose of lewis structure s is to show the arrangement of valence electrons around atoms in a molecule or compound. Web the final lewis structure of nof is: Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Draw lewis structures for the following polyatomic ions: Let's do the nof lewis. As nitrogen is the least. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Web step 1: Find the total valence electrons in nof molecule in order to find the total valence electrons in a nof molecule, first of all you should know the valence electrons present in nitrogen. Video answer this problem has been solved! Web introduction draw the lewis structure of nof (nitrosyl fluoride) chemistnate 248k subscribers subscribe 4.5k views 2 years ago nof is actually onf, since nitrogen has a higher bonding. As nitrogen is the least. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Calculate the total number of valence. Calculate the total number of valence electrons. Include all lone pairs of electrons. O has 1 lone pair, and f has 3 lone pairs. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Lewis structure is a simplified graphic presentation showing the valence electrons around atoms bonded together to make a molecule. Web in the lewis structure for nof there are a total of 18 valence electrons. The lewis structure of nof is drawn by determining the total valence electrons, arranging the atoms with the least electronegative in the center, depicting single bonds between the central and surrounding atoms, and distributing the remaining electrons as lone pairs. Web definition and purpose the. Here, the given molecule is nof. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Valence electrons are the outermost electrons in an atom and are involved in chemical bonding. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and molecular. In order to draw the lewis. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and molecular geometries. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Clo3 each o atom is bonded to the cl. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the main lewis structure of nof. For nitrogen (group 15 element), number of valence. Web definition and purpose the main purpose of lewis structure s is to show the arrangement of valence electrons around atoms in a molecule or compound. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure Include all lone pairs of electrons. To draw the lewis structure of nof we first need to choose a central atom. Web in this task, we need to draw the main lewis structure of n o f \ce{nof} nof using dot notation for nonbonding electrons and bonds for bonding electrons. Lewis structure is a simplified graphic presentation showing the valence electrons around atoms bonded together to make a molecule. Let's do the nof lewis structure. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. Enter the number of bonding electrons followed by the number of nonbonding electrons in the dot structure of this molecule separated by a comma (e.g., 1,2) part b draw the main lewis structure of nof.
How to draw lewis structures for NOF in 60s! Dr K shorts YouTube
Solved Part B Draw the main Lewis structure of NOF. Draw
Solved Part B Draw the main Lewis structure of NOF Draw
Solved Part B Draw the main Lewis structure of NOF Draw

NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube

draw the main lewis structure of nofnof. darnelllemmings
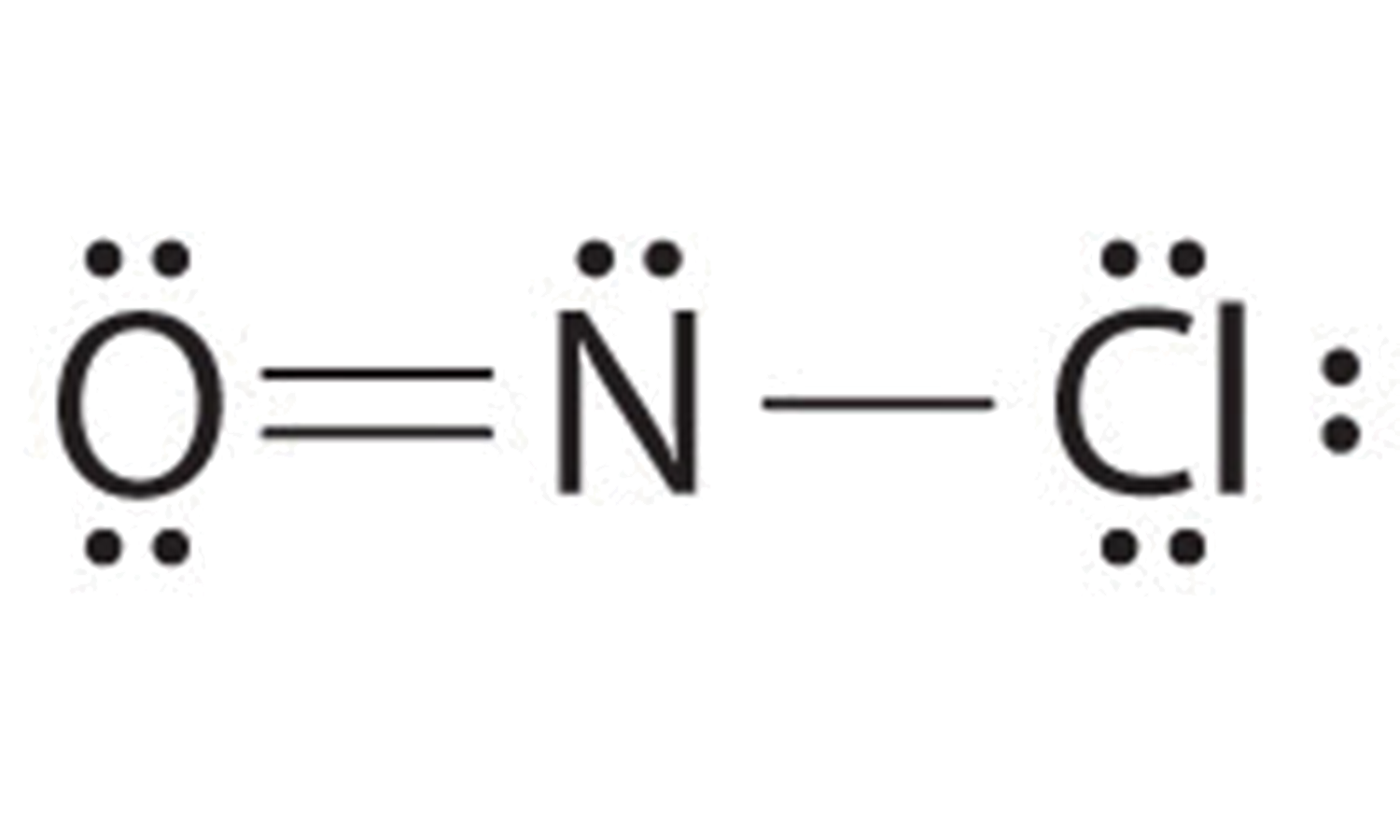
Main Lewis Structure Of Nof
Structure and Geometry The NOF example YouTube

Draw the main lewis structure of nof. draw nonbonding electrons using

Draw The Main Lewis Structure Of Nof Fotodtp
Web The Total Number Of Valence Electrons Available For Drawing The Nitrosyl Fluoride (Nof) Lewis.
Now Counting The Number Of Valence Electrons In The Molecule:
Web In The Lewis Structure For Nof There Are A Total Of 18 Valence Electrons.
With Two Bonding Pairs And Two Lone Pairs, The Oxygen Atom Has Now Completed Its Octet.
Related Post: