Draw The Product Of The Williamson Ether Synthesis Shown.
Draw The Product Of The Williamson Ether Synthesis Shown. - One important procedure, known as the williamson ether synthesis, proceeds by an s n 2 reaction of an alkoxide nucleophile. The williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by alexander williamson in 1850. Web the general method for the synthesis of ether is williamson ether synthesis, involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion. Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. Ch chi он this problem has been solved! Web this organic chemistry video tutorial provides a basic introduction into the williamson ether synthesis reaction mechanism. Web i read that, and was wondering that in the first reaction shown they have used a tertiary butoxide where ether is formed because there is a primary alkyl halide. Create oscersketch answer 1 reaction of the ether shown with one equivalent of hbr. Question 1 draw the product of the williamson ether synthesis shown. Write an equation to show how an ether can be prepared by the. Create oscersketch answer 1 reaction of the ether shown with one equivalent of hbr. Web i read that, and was wondering that in the first reaction shown they have used a tertiary butoxide where ether is formed because there is a primary alkyl halide. Web identify silver(i). English chemist alexander williamson first. One important procedure, known as the williamson ether synthesis, proceeds by an s n 2 reaction of an alkoxide nucleophile. Web the general method for the synthesis of ether is williamson ether synthesis, involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion. This reaction is important in the. This reaction is important in the history of organic chemistry because it helped prove the struc… How could the compound shown below, be made by a williamson ether synthesis? Williamson synthesis uses an s n 2 reaction. Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. Web up to $10 cash back transition metals. This reaction was developed by alexander williamson in 1850. Web a substitution reaction occurring between an oxygen nucleophile and an organohalide electrophile producing an ether product is known as a williamson ether synthesis,. English chemist alexander williamson first. Web science chemistry chemistry questions and answers 2. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide. Ch chi он this problem has been solved! English chemist alexander williamson first. Question 1 draw the product of the williamson ether synthesis shown. Web the williamson ether synthesis is an sn2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. Create oscersketch answer 1 reaction of. Web the general method for the synthesis of ether is williamson ether synthesis, involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion. This reaction was developed by alexander williamson in 1850. Williamson synthesis uses an s n 2 reaction. Web i read that, and was wondering that in the first reaction shown they. Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. Ch chi он this problem has been solved! Question 1 draw the product of the williamson ether synthesis shown. Web draw the mechanism and show the intermediates/products synthesis reaction. The williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a. How could the compound shown below, be made by a williamson ether synthesis? Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. Web science chemistry chemistry questions and answers 2. Web this organic chemistry video tutorial provides a basic introduction into the williamson ether synthesis reaction mechanism. Write an equation to show how an. The williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). (10 points) of the following williamson ether nah он. English chemist alexander williamson first. Write an equation to show how an ether can be prepared by the. Web identify silver(i) oxide as a reagent which can be used in a williamson. Web i read that, and was wondering that in the first reaction shown they have used a tertiary butoxide where ether is formed because there is a primary alkyl halide. Web this organic chemistry video tutorial provides a basic introduction into the williamson ether synthesis reaction mechanism. (10 points) of the following williamson ether nah он. Web a substitution reaction. This reaction was developed by alexander williamson in 1850. Web i read that, and was wondering that in the first reaction shown they have used a tertiary butoxide where ether is formed because there is a primary alkyl halide. One important procedure, known as the williamson ether synthesis, proceeds by an s n 2 reaction of an alkoxide nucleophile. Web draw the mechanism and show the intermediates/products synthesis reaction. Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. Write an equation to show how an ether can be prepared by the. How could the compound shown below, be made by a williamson ether synthesis? Web the williamson ether synthesis is an sn2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. Web williamson ether synthesis is a common organic chemistry reaction that makes ethers from oxides (or alcohols) and alkyl halides. Web a substitution reaction occurring between an oxygen nucleophile and an organohalide electrophile producing an ether product is known as a williamson ether synthesis,. Williamson synthesis uses an s n 2 reaction. This reaction is important in the history of organic chemistry because it helped prove the struc… Question 1 draw the product of the williamson ether synthesis shown. Web identify silver(i) oxide as a reagent which can be used in a williamson synthesis. The williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). It contains plenty of examples and.
Williamson Ether Synthesis Chemistry Steps

Draw the mechanism for the Williamson ether reaction using mcresol and

Williamson Ether Synthesis EXPLAINED w/examples YouTube
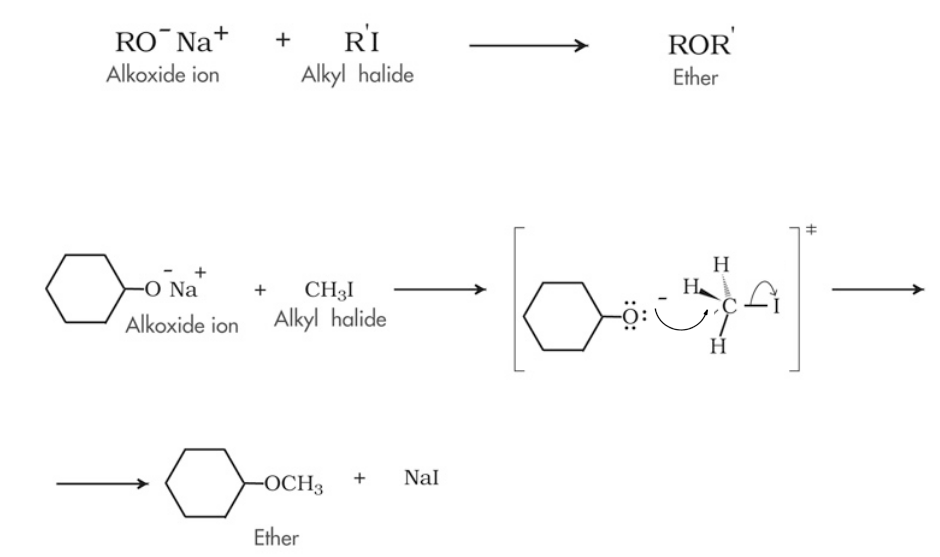
9.5 Williamson ether synthesis Chemistry LibreTexts

The Williamson ether synthesis is an organic reaction, forming an ether

Williamson Ether Synthesis Organic chemistry, Ethereal, Basic facts
![[Solved] Product of Williamson Synthesis 9to5Science](https://i.stack.imgur.com/xHY1j.jpg)
[Solved] Product of Williamson Synthesis 9to5Science

Draw the mechanism for the williamson ether reaction using mcresol and
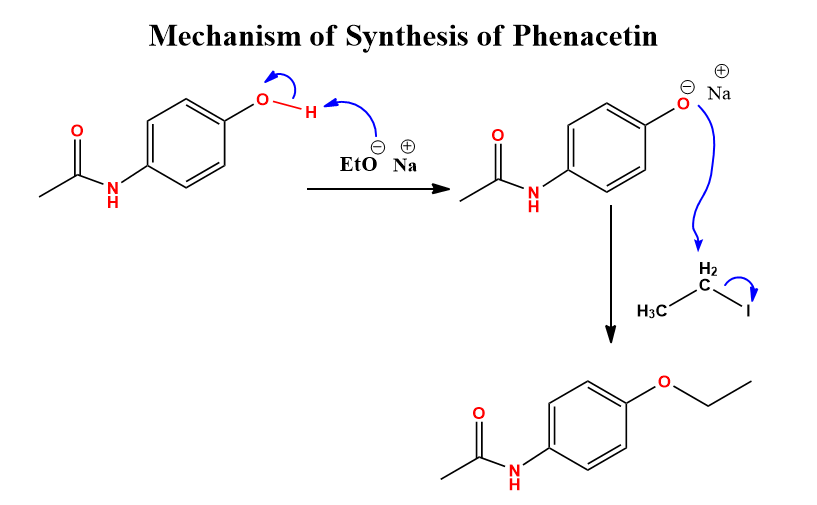
Williamson ether synthesis simple mechanism, 3 examples Chemistry Notes
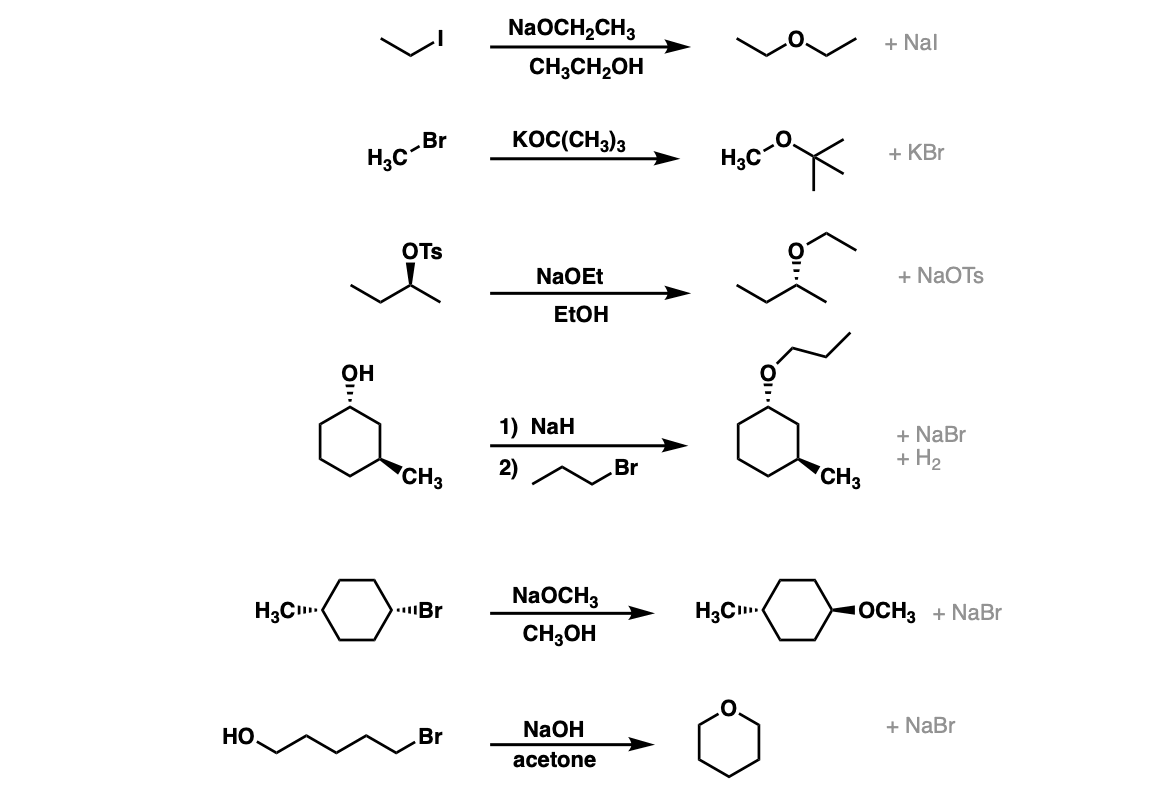
SN2 reaction of alkoxide ions with alkyl halides to give ethers
Ch Chi Он This Problem Has Been Solved!
Web The General Method For The Synthesis Of Ether Is Williamson Ether Synthesis, Involves Nucleophilic Displacement Of A Halide Ion Or Other Good Leaving Group By An Alkoxide Ion.
Web Draw The Target Ether, Identify The Two Groups Attached To Oxygen, And Recall The Limitations Of The Two Methods For Preparing Ethers.
(10 Points) Of The Following Williamson Ether Nah Он.
Related Post: