Drawing Covalent Bonds Worksheet
Drawing Covalent Bonds Worksheet - Draw the lewis diagram for the covalent bond in the h 2 molecule. Web draw lewis structures for covalent compounds. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Sharing 1 pair of electrons creates a singlecovalent bond, sharing 2 pairs of electrons creates a double covalentbond, and sharing 3 pairs of electrons creates a triple covalent bond. Use lewis dot structures to show the ionic bonding in the. Web the covalent bonding worksheet covers the following topics: Web covalent bonds involvesharing pairs of electronsbetween two atoms so that each atom gainsa full outer valence shell. H 3 nbf 3 (2 marks) 4. Extension draw a cluster diagram for each type of bond. The first thing that you must do is to determine the number of electrons available for the formula. Circle the unpaired electrons that will be shared between the elements. There's no need to solve the worksheet yourself (unless you want to, that is), the included answer sheet shows the correct solution and makes marking your students' work a piece of cake. For example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell:. Describe the type of bonding that occurs in the compound. Web students will practice drawing covalent bonds with this worksheet. Circle the unpaired electrons that will be shared between the elements. The next step is to determine which atom will be in the center of the molecule. Web draw lewis structures for covalent compounds. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b atom. Web step 1: Students should be familiar with drawing lewis diagrams of atoms. Electrostatic forces in ionic bonds; Below is a completed example: Covalent bonds and molecular structure how are ionic bonds and covalent bonds different? Draw the electron dot structure. Identifying ionic, covalent and metallic bonds from diagrams; Sharing electrons in covalent bonds; Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b atom. Metal atoms lose electrons to form cations. Sharing 1 pair of electrons creates a singlecovalent bond, sharing 2 pairs of electrons creates a double covalentbond, and sharing 3 pairs of electrons creates a triple covalent bond. Web the bonding worksheets cover the following topics: The first thing that you must do is to determine the number of electrons available for. Web draw lewis structures for covalent compounds. Describe the type of bonding that occurs in the compound. Students should be familiar with drawing lewis diagrams of atoms. Sharing electrons in covalent bonds; Draw the lewis diagram for the covalent bond in the h 2 molecule. Draw the lewis diagram for the covalent bond in the br 2 molecule. Web draw a lewis dot diagram for each element listed. The next thing that you must do is to draw an. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Follow your teacher’s directions to complete each covalent. Web chapters 6 and 7 practice worksheet: Hydrogen atoms are only able to. Sharing electrons in covalent bonds; Write down what atoms you need and how many of them step 3: Drawing covalent bonds use the step by step method below to answer the questions that follow. Web covalent bonds involvesharing pairs of electronsbetween two atoms so that each atom gainsa full outer valence shell. Identify the type(s) of bond(s) found in the following molecules: Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b atom. Describe the relationship between the length of a bond and the strength of that bond.. Web draw the lewis dot structure for each of the following polyatomic ions: Describe the type of bonding that occurs in the compound. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Therefore, all electron clouds around the central atom. Metal atoms lose electrons to form cations. The presence of positive metal ions and negative electrons in metallic bonds; Students should be familiar with drawing lewis diagrams of atoms. Web the most common examples are the covalent compounds of beryllium and boron. Boron commonly makes only three covalent bonds, resulting in only six valence electrons around the b atom. Types of elements involved in ionic. Web define covalent bond. Web students will practice drawing covalent bonds with this worksheet. Identifying ionic, covalent and metallic bonds from diagrams; The first thing that you must do is to determine the number of electrons available for the formula. Use lewis dot structures to show the ionic bonding in the. The next thing that you must do is to draw an. H 3 nbf 3 (2 marks) 4. Nh 4 + (2 marks) 3. Web draw a lewis dot diagram for each element listed. Web draw lewis structures for covalent compounds. The presence of ions in ionic bonds;
WS Drawing Covalent Bond Diagrams PDF Name Class Date DRAWING

Learn About Covalent Bonding With This Informative Worksheet Style

Covalent Bonding Questions and Revision MME

️Drawing Covalent Bonds Worksheet Free Download Goodimg.co
Covalent Bond Worksheet PDF

Covalent Bonds Worksheets Middle School
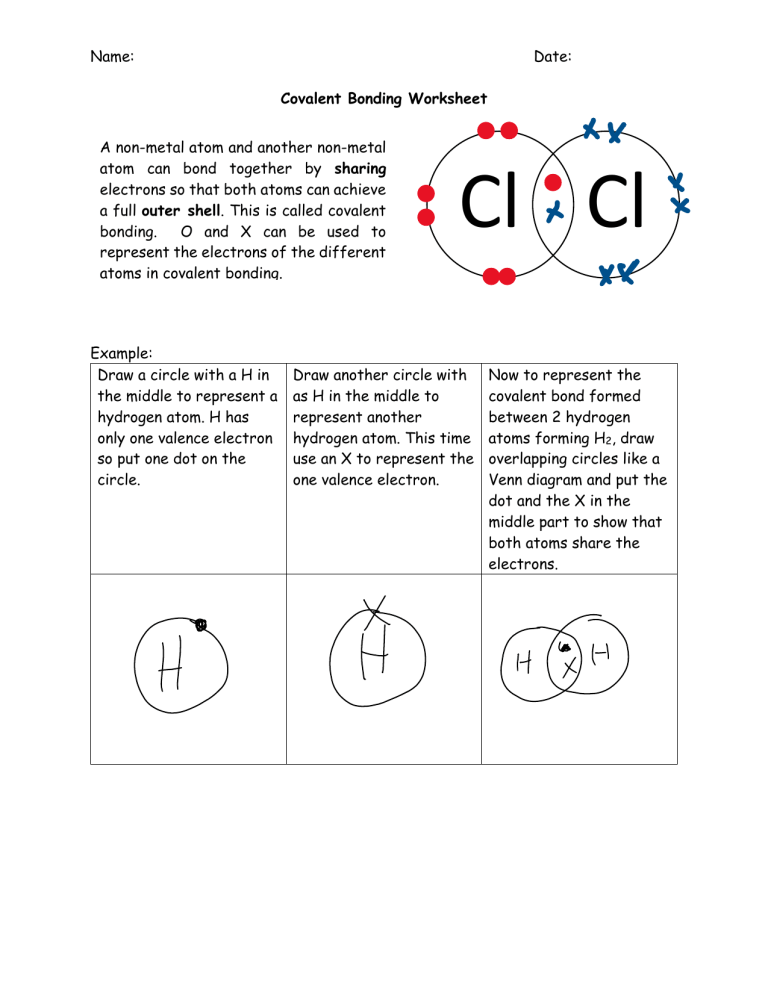
Covalent bonding worksheet

Drawing Covalent Bonds Worksheet

Covalent bond worksheet Covalent bonding worksheet, Covalent bonding
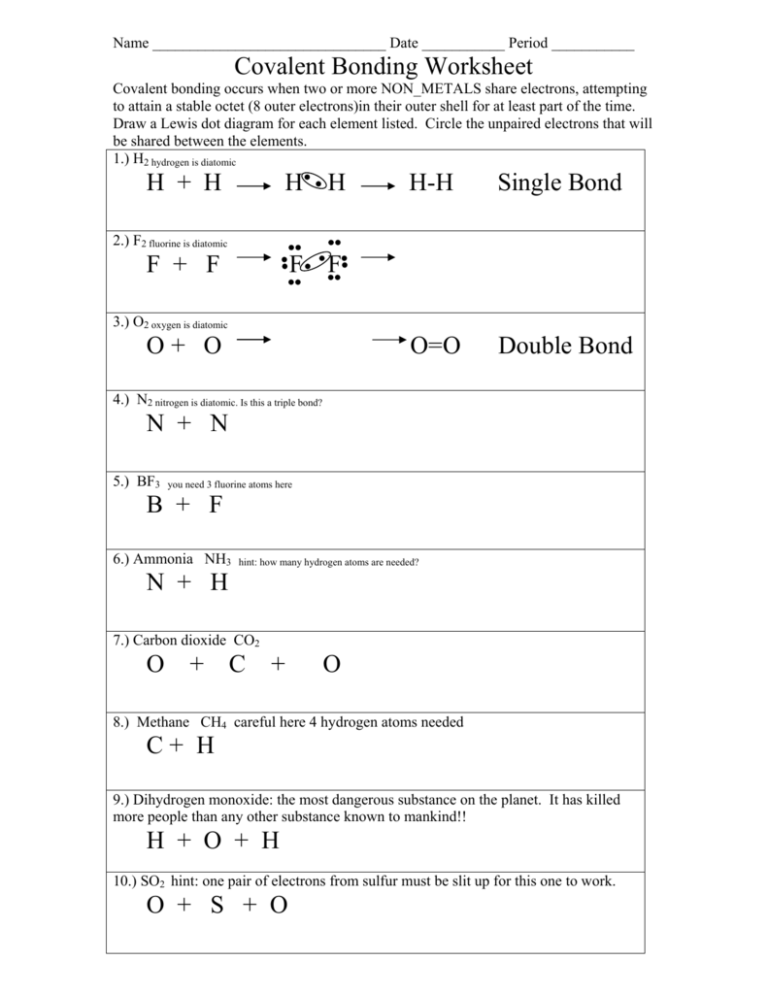
Covalent Bonding Worksheet
• Interpreting Diagrams Representing Covalent Bonds • Sharing Electrons In Covalent Bonds • Types Of Elements Involved In Covalent Bonds.
Draw The Lewis Diagram For The Covalent Bond In The Hcl Molecule.
Therefore, All Electron Clouds Around The Central Atom.
Lesson Summary The Octet Rule In Covalent Bonding Covalent Compounds Are Most Stable When Each Atom Has Eight Electrons.
Related Post:
