Drawing The Mo Energy Diagram For A Period 2 Homodiatom
Drawing The Mo Energy Diagram For A Period 2 Homodiatom - O structure and bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the b2+ molecular ion. You’ll see the molecule is not possible, but none the less. That must mean that each atom has two 1s electrons of its own, for a total of four. Web chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the hcz molecular ion: `2` electrons go into the `sigma_2s`, `2` into the `sigma_2s^*`, `2` into the `sigma_2p`, `4` into the `pi_2p`, and. Be sure your diagram contains all of the electrons in the ion, including any core electrons. This will be sigma star 2 s if we are in the orbital. Be sure your diagram contains all of the electrons in the ion, including any core electrons. The number of molecular orbitals produced is the same as the number of atomic orbitals used to create them (the law of conservation of. When those four electrons are filled into the mo diagram from the bottom up, they will occupy both the bonding σ 1s and the antibonding σ 1s *. They are the same energy because the are the same except rotated 90°. So when the boron is in resting state, the two electrons will lie over here and from this, they will come in this or vital. Web 196 views 2 years ago. Now, fill in the electrons from the bottom up. Be sure your diagram contains all of. Energy 1 ح di х ? Web chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the h2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. We put the same number of valence electrons we had in the aos. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Then you fill those lines (molecular orbitals) with 4 electrons (two from each beryllium) there’s you’re mo diagram. This will be the sigma star 2 s if we see the orbital or if we're in. This will be sigma star 2 s if we are in the orbital. Web chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the hcz molecular ion: Web since we're doing the mo diagram for `o_2`, we have to use the `o_2` mo diagram which features flipped `pi_2p` and. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. At last sigma 2 pitso, 2 will be further proceeded by sigma 2 and i begin. Web in o 2 and f 2, there is a crossover of the sigma and the pi ortbials: Drawing. Fill out the valence electrons. At last sigma 2 pitso, 2 will be further proceeded by sigma 2 and i begin. Web in the context of mo, suppose we do have 2s electrons. You’ll see the molecule is not possible, but none the less. Explanation check doo 20 f3 f2 f4 f5 f7 show transcribed image text Web since we're doing the mo diagram for `o_2`, we have to use the `o_2` mo diagram which features flipped `pi_2p` and `sigma_2p` orbitals. This will be sigma star 2 s if we are in the orbital. So we are draw drawing that toupee and two with structure that energy will be increasing like this. The number of molecular orbitals. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. They are the same energy because the are the same except rotated 90°. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday. So we are draw drawing that toupee and two with structure that energy will be increasing like this. Sigma 2 and sigma 2 pitso will be followed by sigma 2 and sigma 2. Be sure your diagram contains all of the electrons in the. This will be sigma star 2 s if we are in the orbital. Be sure your. So when the boron is in resting state, the two electrons will lie over here and from this, they will come in this or vital. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Now, fill in the electrons from the bottom up. Web drawing the mo energy diagram for a period 2. When those four electrons are filled into the mo diagram from the bottom up, they will occupy both the bonding σ 1s and the antibonding σ 1s *. That means the highest number of orbit is too. Web drawing the mo energy diagram for a period 2 homodiatom. So we are draw drawing that toupee and two with structure that energy will be increasing like this. Drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the n2+ molecular ion. This will be sigma star 2 s if we are in the orbital. So when the boron is in resting state, the two electrons will lie over here and from this, they will come in this or vital. Now, fill in the electrons from the bottom up. Fill out the valence electrons. Web in o 2 and f 2, there is a crossover of the sigma and the pi ortbials: Sigma 2 and sigma 2 pitso will be followed by sigma 2 and sigma 2. O electronic structure and chemical bonding drawing the mo energy diagram for a period 2 homodiatom draw the molecular orbital (mo) electron diagram for the b, molecular ion. The berylium 2 molecule has a negative charge, so we need to draw a diagram for it. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Be sure your diagram contains all of the electrons in the ion, including any core electrons. `2` electrons go into the `sigma_2s`, `2` into the `sigma_2s^*`, `2` into the `sigma_2p`, `4` into the `pi_2p`, and.MO Diagrams for First Row Diatomic Molecules Chemistry LibreTexts
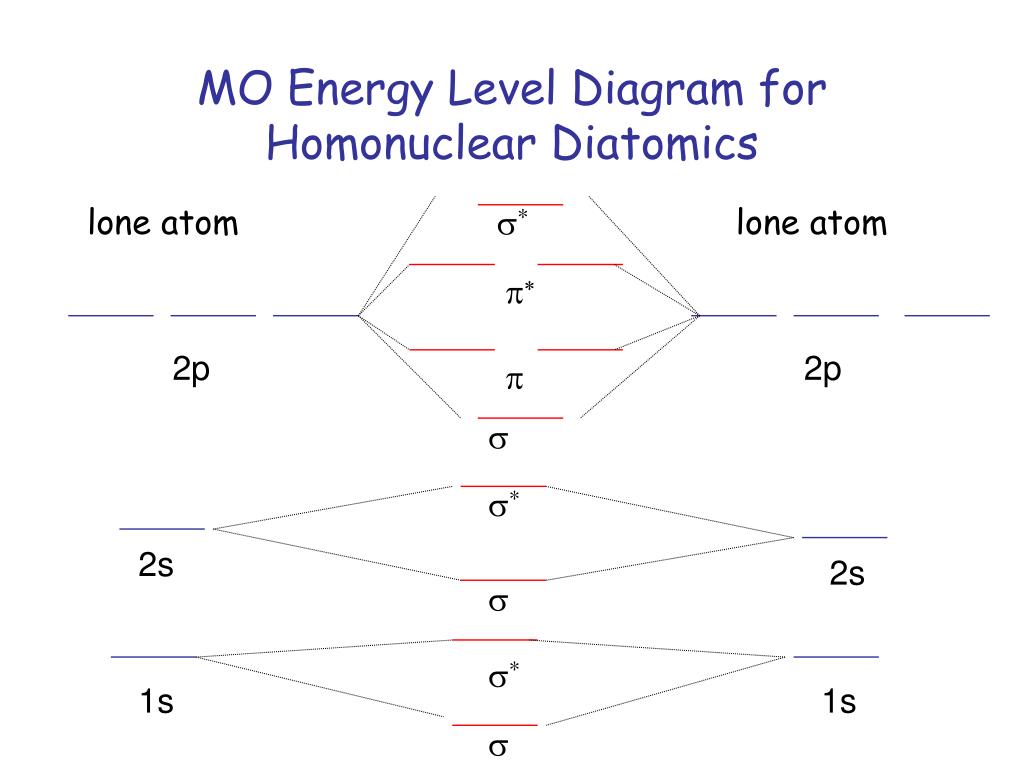
PPT MO Theory PowerPoint Presentation, free download ID685279
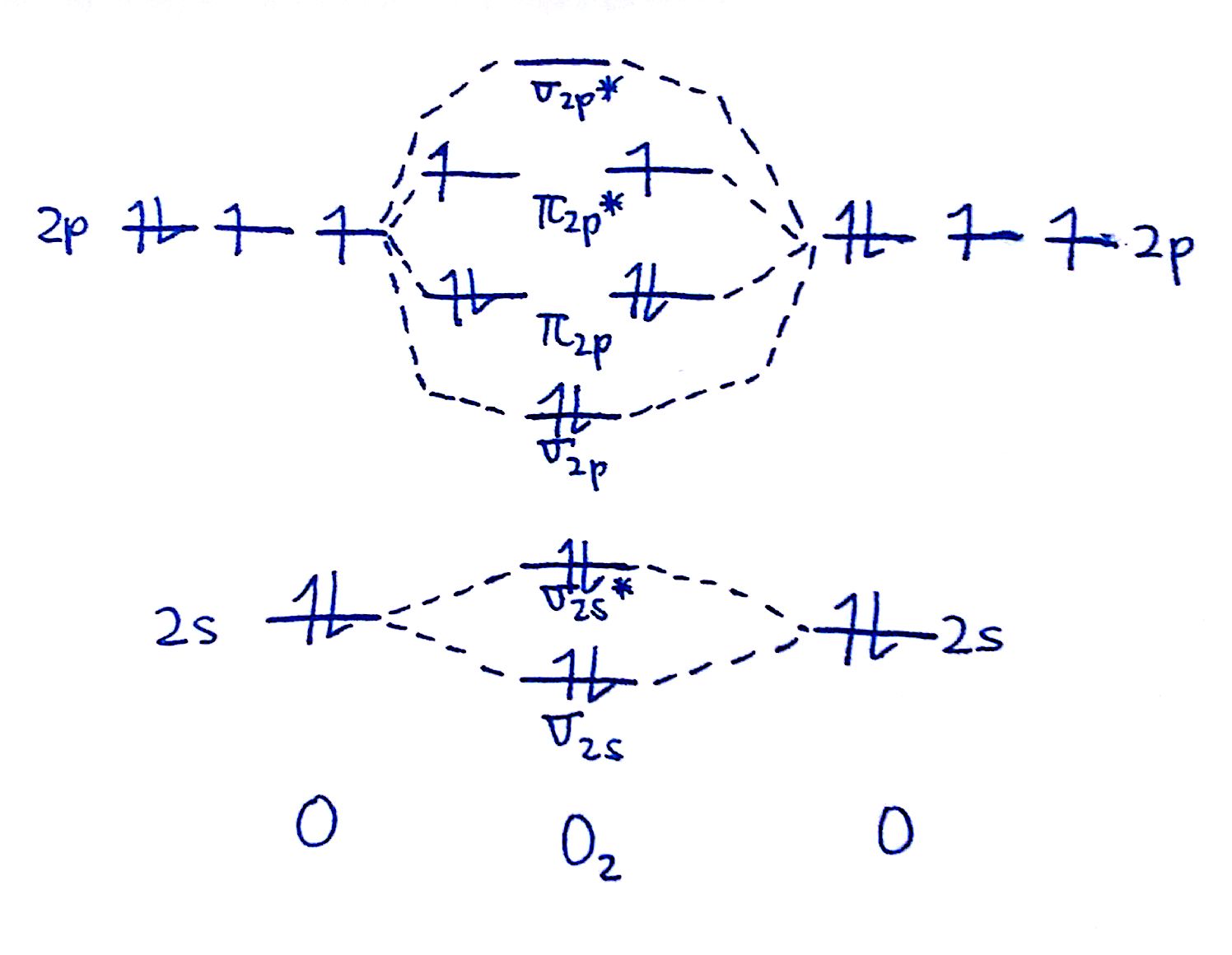
MO Diagrams

9.3e Drawing the MO energy diagram for a Period 2 homodiatom YouTube

Drawing the MO energy diagram for a Period 2 homodiatom YouTube

Aleks Drawing the MO energy diagram for a period 2 homodiatom YouTube

ALEKS Drawing the MO energy diagram for a Period 2 homodiatom YouTube

Drawing the MO energy diagram for a Period 2 homodiatom YouTube
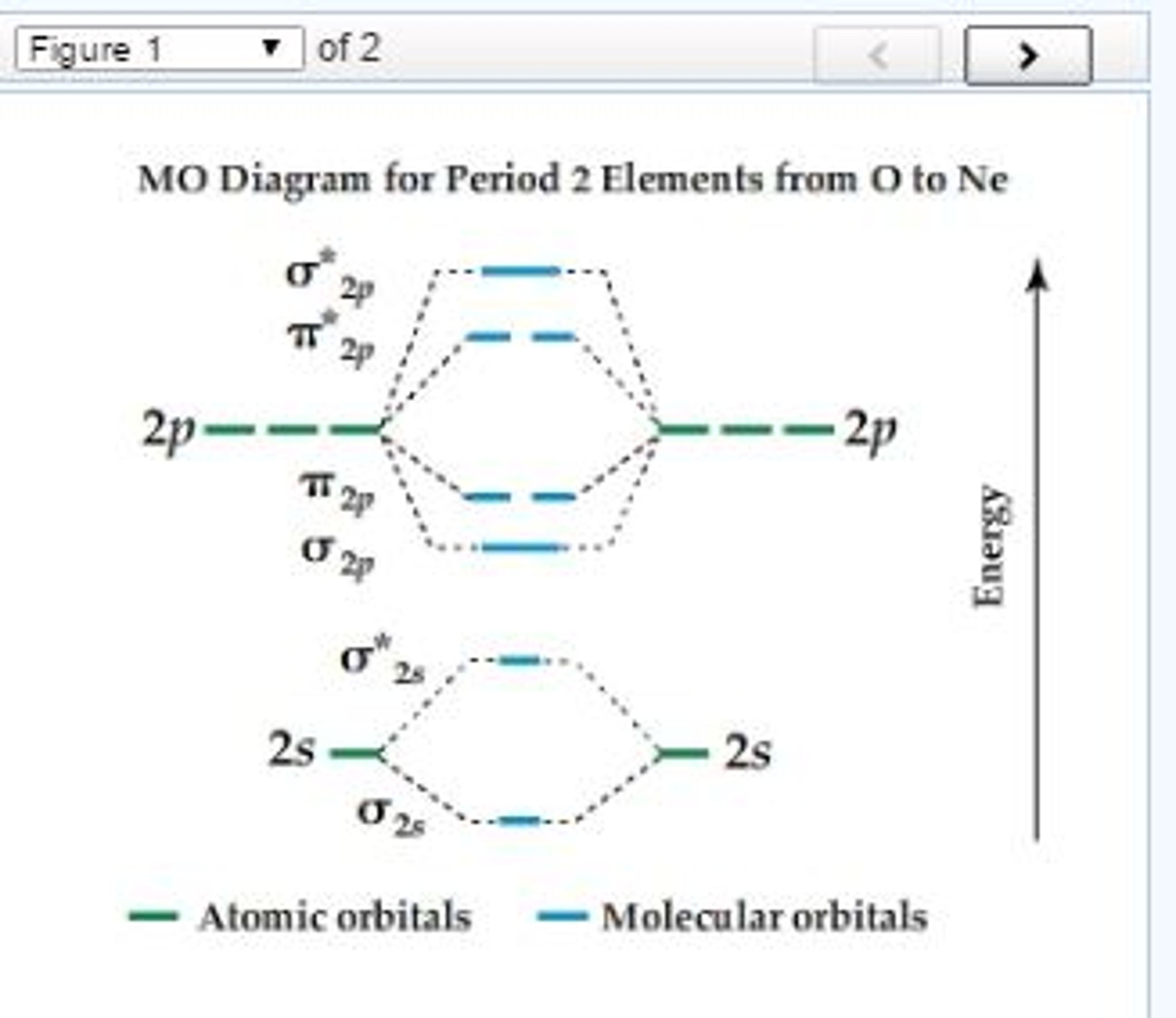
Solved MO Diagram For Period 2 Elements From O To Ne Arra...
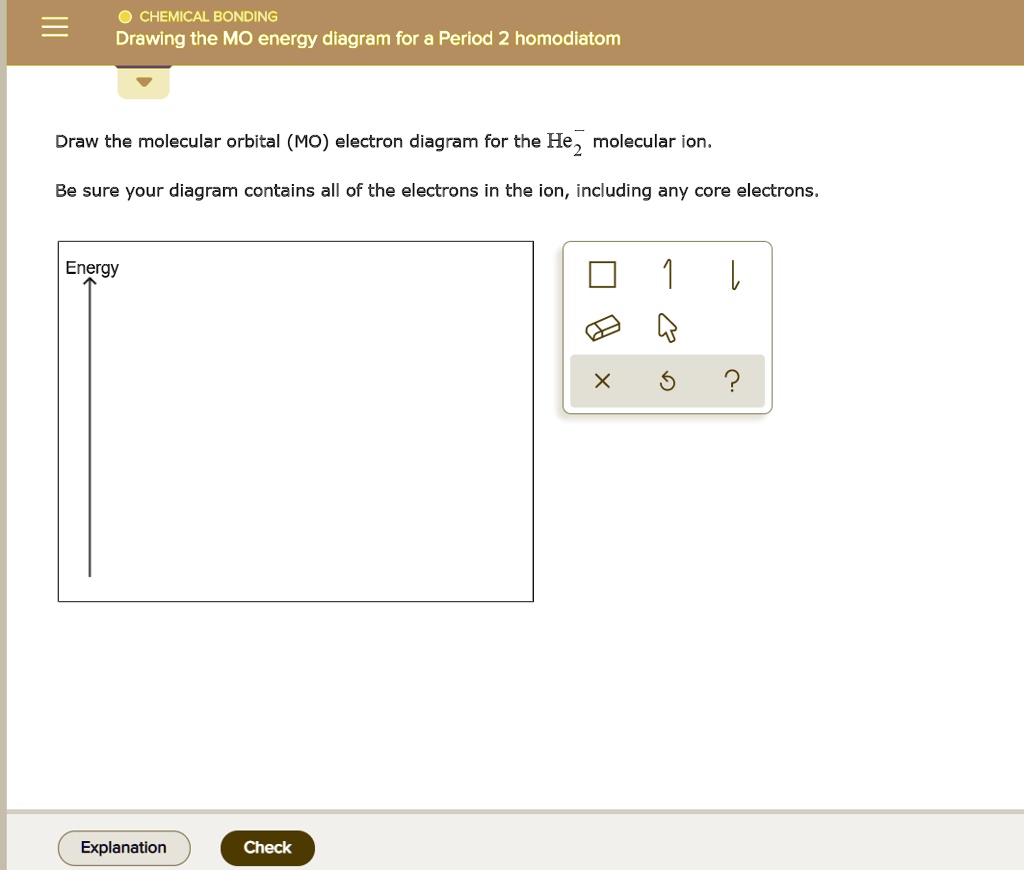
CHEMICAL BONDINGDrawing the MO energy diagram for a P… SolvedLib
Be Sure Your Diagram Contains All Of The Electrons In The Ion, Including Any Core Electrons.
Information From The Mo Diagram Justify O2'S Stability And Show That It's Bonding Order Is 2.
One Is To Two Ways To To Be One.
Energy Explanation Check O Type Here To Search.
Related Post: