Hcn Perspective Drawing
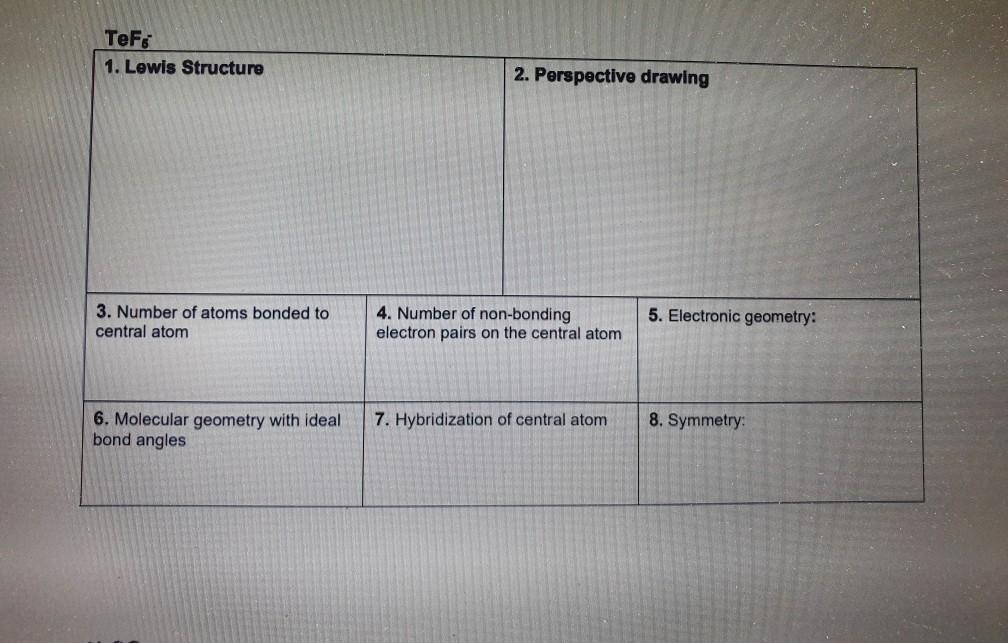
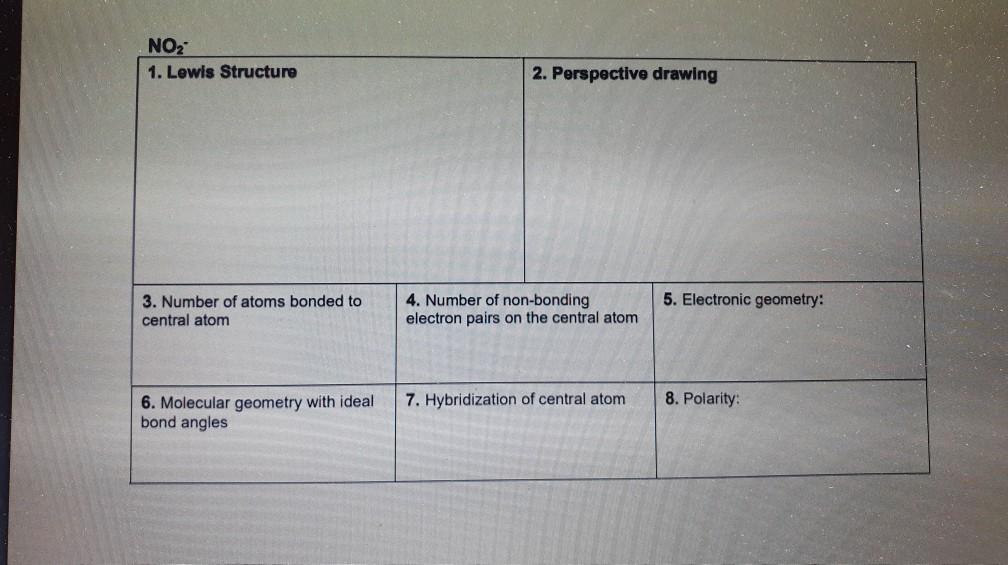
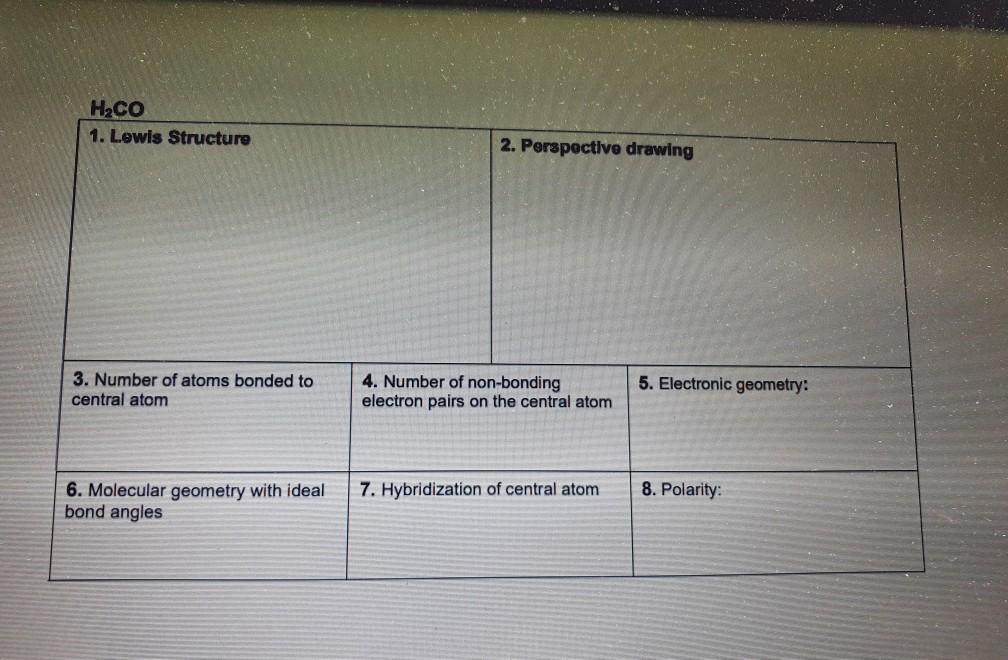
Hcn Perspective Drawing - To determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions. Number of atoms bonded to central atom 4. • how to draw lewis. #of lone electron pairs 6. Web therefore, the very first step while drawing the lewis structure of hcn is to count the total valence electrons present in the concerned elemental atoms. Web here's how to do it. Web how to draw lewis structure for hcn? To calculate the valence electron of each atom in hcn, look for its periodic group from the periodic table. Electronic geometry central atom atom 7. How to draw a lewis structure for hcn? Hybridization of central atom ch&oh You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To draw lewis structures (both projection and perspective drawings) for each of these molecules and ions. Number of atoms bonded to central atom 4. Web in chem 101a, we will focus on drawing lewis structures of molecules and polyatomic. Chemistry learning made easy.this tutorial will help you deal with the lewis structure and. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Number of atoms bonded to central atom 4. • how to draw lewis. Such molecules are very common, and they provide a foundation for understanding. To begin, you must know two essential rules for drawing. We're going to talk about the livius structure of different radicals and molecules. Web how to draw lewis structure for hcn? Electronic geometry atom central atom on central atom 7. Add these electrons to give every atom an octet you nave to put a triple bond between c and n. 100% (3 ratings) transcribed image text: #of atoms bonded to 5. Electronic geometry central atom atom 7. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. To determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions. We'll also compare hnc to hcn and discuss why both are of inter. He was obsessed with the vanishing point, and also birds (cute!). Electronic geometry central atom atom 7. We're going to talk about the. Determine the total valence electrons to start, we need to determine the total number of valence electrons in the hcn molecule. To draw lewis structures (both projection and perspective drawings) for each of these molecules and ions. Molecular geometry with ideal bond angles 7. 100% (3 ratings) transcribed image text: Hybridization of central atom 8. There is one bond between h and c and three bonds between c and nitrogen. The valence electrons present in different elemental atoms of a molecule can be readily determined by identifying those atoms in the periodic table. Hybridization of this problem has been solved! The structure of its life is similar to this. Molecular geometry with ideal bond angles. Electrons on the nitrogen atom. To draw lewis structures (both projection and perspective drawings) for each of these molecules and ions. The compound has sp hybridization. The valence electrons present in different elemental atoms of a molecule can be readily determined by identifying those atoms in the periodic table. Molecular geometry with ideal bond angles. To calculate the valence electron of each atom in hcn, look for its periodic group from the periodic table. • how to draw lewis. #of atoms bonded to 5. # of atoms bonded to 5. Number of atoms bonded to central atom. 1.1k views 3 years ago chemistry. We'll also compare hnc to hcn and discuss why both are of inter. The compound is polar in nature. How to draw a lewis structure for hcn? Number of atoms bonded to central atom 4. He was obsessed with the vanishing point, and also birds (cute!). To begin, you must know two essential rules for drawing. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side. There is one lone pair of; Hybridization of central atom ch&oh The compound is polar in nature. Web here's how to do it. We will be going through molecules with steric number 2, 3 and 4. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. The second step is to calculate the hcn hybridization, and the third step is to give perfect notation for the hcn molecular geometry. The molecular geometry of hcn is linear. # of lone electron pairs on central atom 6. Hcn lewis dot structure by counting valence electrons on the carbon and nitrogen atom. #of lone electron pairs 6. Web therefore, the very first step while drawing the lewis structure of hcn is to count the total valence electrons present in the concerned elemental atoms. Number of atoms bonded to central atom 4.Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.

Lewis Dot Diagram Of Hcn
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
The 3D Geometries We Will Be Drawing Are Linear.
Web How To Draw Lewis Structure Of Hcn Step 1:
Electronic Geometry Atom Central Atom On Central Atom 7.
The First Step Is To Sketch The Molecular Geometry Of The Hcn Molecule, To Calculate The Lone Pairs Of The Electron In The Central Carbon Atom;
Related Post: