How To Draw An Atom With Protons Neutrons And Electrons
How To Draw An Atom With Protons Neutrons And Electrons - Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: With an atomic mass of 14, when we subtract the six protons, the number of. Dalton's atomic theory explained a lot about matter, chemicals, and chemical reactions. What element is represented by the diagram? This is where the protons and neutrons are located. Web it is called the strong force (really, that is its name). Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Draw two electrons in the first energy level and label them with their charge. Web welcome to my tutorial on how to draw an atom! But an atom must have just the right balance of protons to neutrons to make a stable nucleus. The arrow points to the first energy level. Protons have a positive charge. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web key concepts atoms are made of extremely tiny particles called protons, neutrons, and electrons. Electrons are the particles that orbit around the nucleus in different energy levels. Neutrons are a type of subatomic particle with no charge (they are neutral). Web welcome to my tutorial on how to draw an atom! The filling of the electron shells depends on their orbital. What element is represented by the diagram? Web determine the number of protons and electrons in an atom. The first orbital (an s orbital) can contain only two electrons. Web phet global deib in stem ed donate build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Web the positive charge on a proton is equal in magnitude to the negative charge on an electron. This atom is also balanced. Both neutrons and protons carry the strong force (but electrons do not). Web the protons and neutrons go in the middle of the atom. What element is represented by the diagram? The first orbital (an s orbital) can contain only two electrons. Neutral atoms have equal numbers of protons and. Web o protons = 6: Both neutrons and protons carry the strong force (but electrons do not). As long as it’s carbon it has six protons. Protons and neutrons are in the center of the atom, making up the nucleus. The charge on the proton and electron are exactly the same size but. Draw six neutrons in the nucleus of the atom. Web draw a cluster of circles at the center to form the nucleus, which consists of neutrons and protons. Web key concepts atoms are made of extremely tiny particles called protons, neutrons, and electrons. Web drawing an atom can be a fun and creative way to explore the different parts of. The first two electrons go into the first energy level. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons. Then play a game to test your ideas! Electrons have a negative. Web atoms smallest particle is a string. However today we know that this is false. What element is represented by the diagram? Beryllium greg robson/cc by 2.0 boron Web draw a cluster of circles at the center to form the nucleus, which consists of neutrons and protons. Web multiple examples, including a detailed explanation of how to complete each. As a result, a neutral atom must have an equal number of protons and electrons. For a neutral atom, the number of protons and the number of electrons are equal. Web you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.. Web it is called the strong force (really, that is its name). For a neutral atom, the number of protons and the number of electrons are equal. With an atomic mass of 14, when we subtract the six protons, the number of. Then play a game to test your ideas! O neutrons = 8 : Web lithium greg robson/cc by 2.0 lithium is the first element in which an additional electron shell is added. Web protons and neutrons are the particles that make up the nucleus of an atom, which is the central part of the atom. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Draw six neutrons in the nucleus of the atom. Protons are a type of subatomic particle with a positive charge. As a result, a neutral atom must have an equal number of protons and electrons. Web it is called the strong force (really, that is its name). Electrons have a negative charge. Web simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons. Protons and neutrons are in the center of the atom, making up the nucleus. Beryllium greg robson/cc by 2.0 boron However today we know that this is false. The charge on the proton and electron are exactly the same size but. Web atoms smallest particle is a string. Web determine the number of protons and electrons in an atom. Web you can use these numbers to calculate the number of protons, neutrons and electrons in an atom.
Atomic Structure Broad Learnings
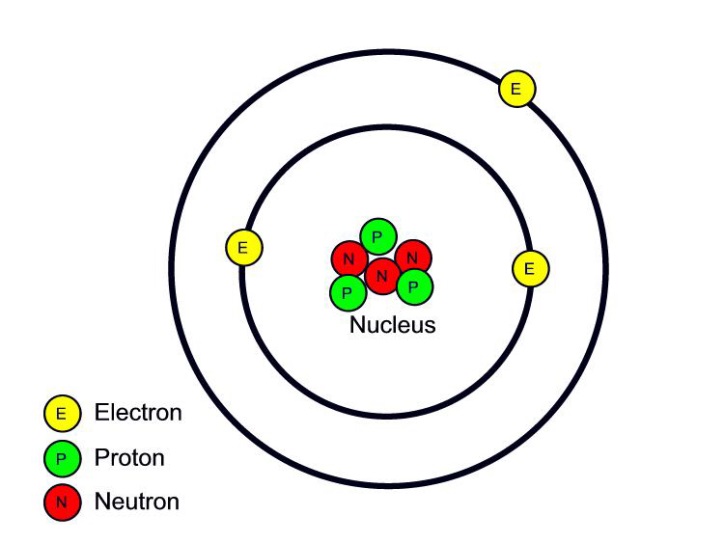
Atomic structure WGHS Junior Science

Learn the Parts of an Atom
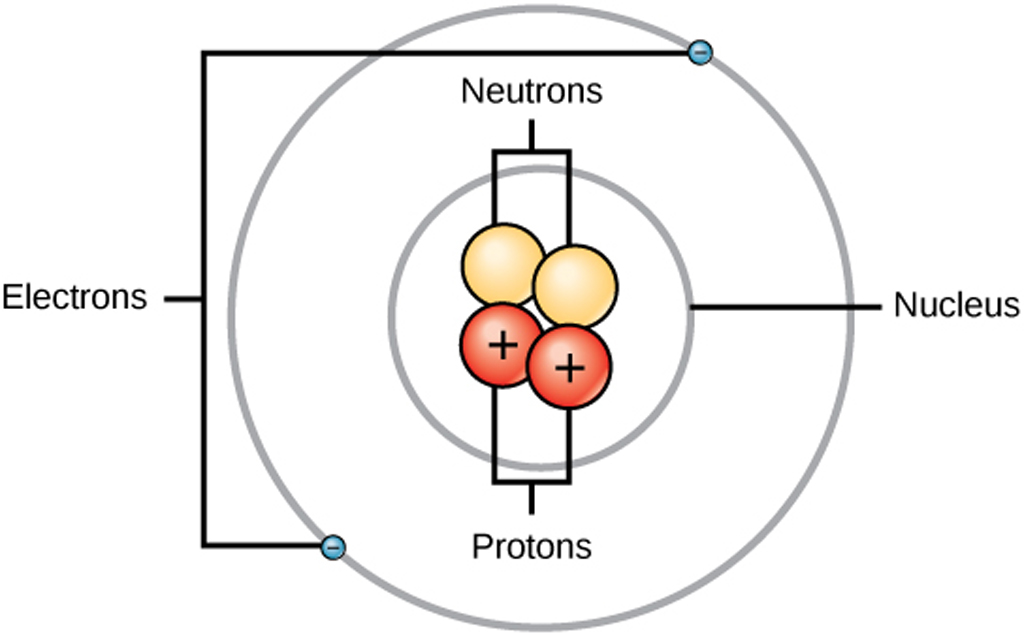
Atom Structure Electronics Tutorial The Best Electronics Tutorial
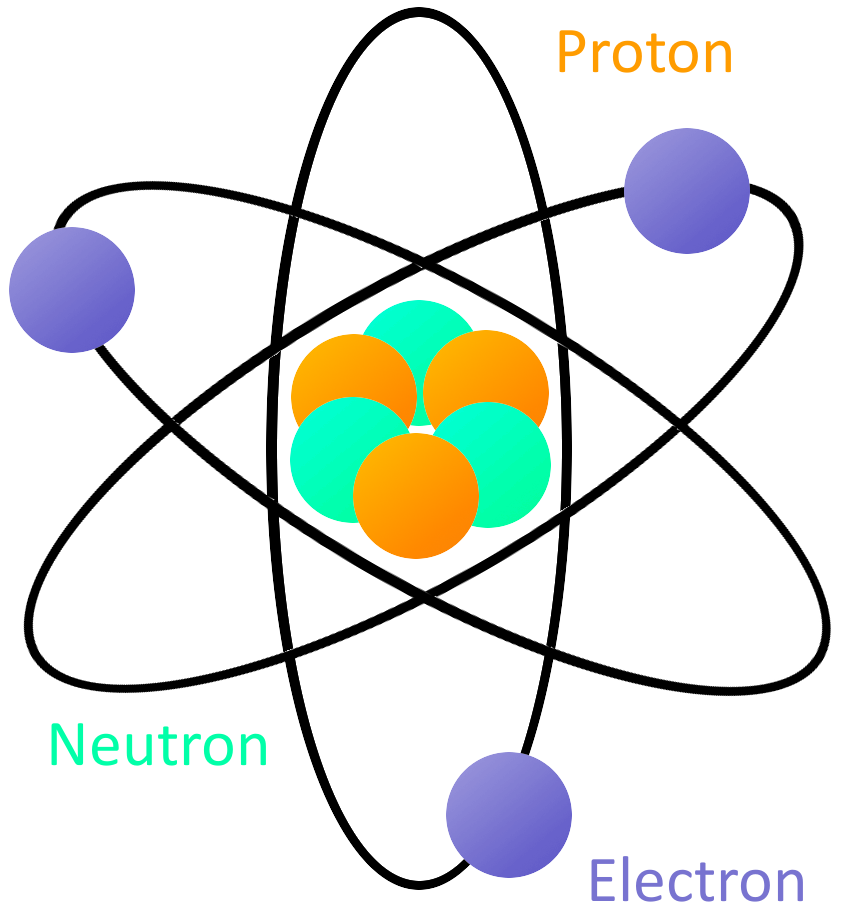
What is Electricity?

Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind

Protons — Structure & Properties Expii
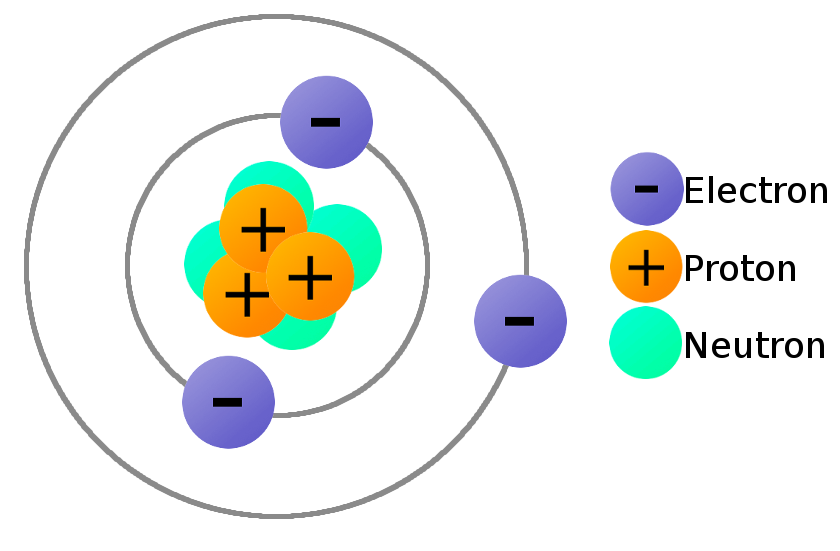
Proton, Electron, Neutron Definition Formula Application
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
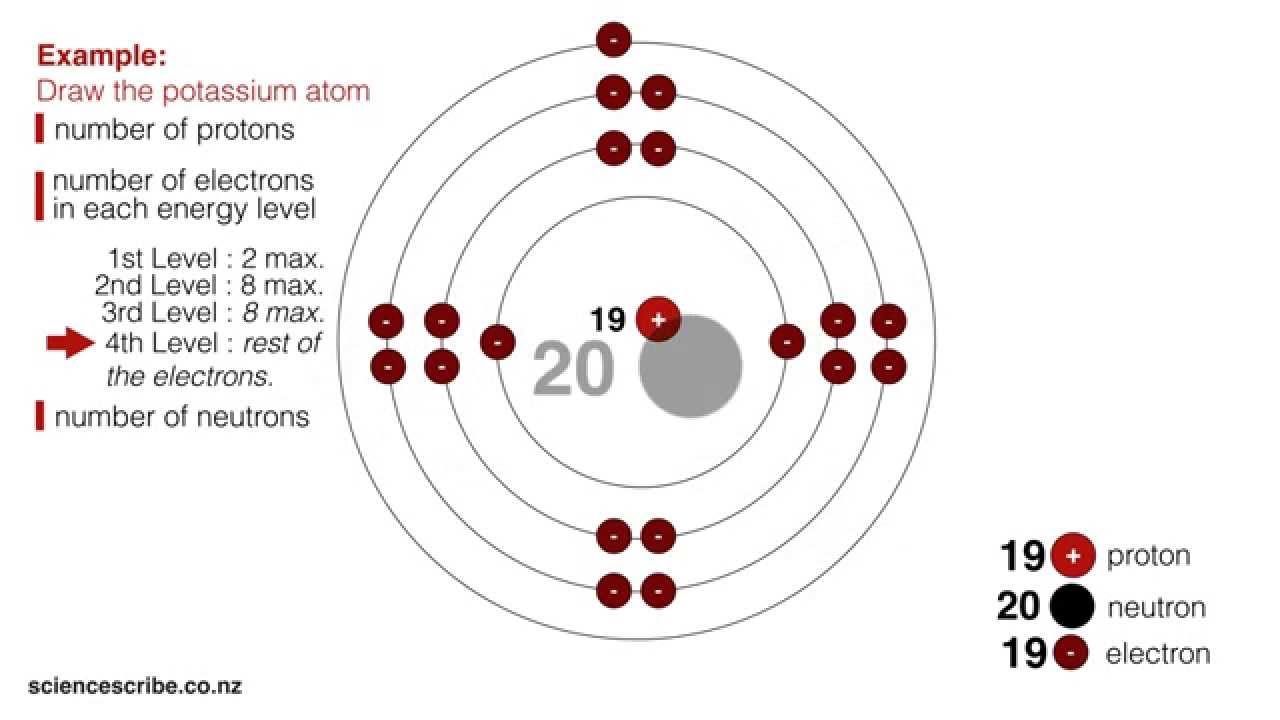
Drawing Atoms (NCEA L1 & Junior Science) YouTube
This Atom Is Also Balanced In Charge So It Also Needs Six Electrons.
The First Two Electrons Go Into The First Energy Level.
The Strong Force Is What Binds The Nucleus Together, By Overcoming The Repulsion Between The Protons.
On A Sheet Of Paper, Draw A Carbon Atom, Then Flip The Card To See If You Drew It Correctly.
Related Post: