How To Draw An Atomic Structure
How To Draw An Atomic Structure - The energy level is determined by the period and the number of electrons is given by the atomic number of the element. In the bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons. Protons, neutrons, and photons a protons, neutrons, and photons positrons,. An electron is a negatively charged particle. Of proton + no of neutrons. Web drawing atomic structures schoolrevision 58 subscribers subscribe 120 share save 19k views 9 years ago in this video i have used the example of sodium, simply because. Hydrogen has 1 proton and no neutrons. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Electrons orbit the nucleus of an atom which is made up of both protons and neutrons. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Web how to draw an atom | atom structure diagram | physics project diagram. The mass number of fluorine is 19 and. A lewis structure also helps to make a prediction about the geometry of a molecule. To indicate they are protons, draw them as circles with plus signs contained inside. Web faqs the advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. Web a lewis structure is a graphic representation of the electron distribution. This is sometimes called the bohr, or the ‘solar system’, model. The structure of the atom. Web draw your protons and neutrons. If you want (or need) to draw a model of an atom, we'll show you how!#atom #atomicstructure #atomicmodel #howtodraw. A lewis structure also helps to make a prediction about the geometry of a molecule. Web 2.8m views 5 years ago. Web part 1 learning the components of an atom 1 learn about the properties of an electron. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Covalent bonds are shown using lines. When constructing a lewis diagram, keep in mind the octet rule, which refers to the. A lewis structure also helps to make a prediction about the geometry of a molecule. Web drawing atomic structures schoolrevision 58 subscribers subscribe 120 share save 19k views 9 years ago in this video i have used the example of sodium, simply because. An electron is a negatively charged particle. Shared pairs of electrons are drawn as lines between atoms,. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. It is total number of proton and neutron present in the nucleus of each atom of an element. The numbers of subatomic particles in an atom can. Web how to draw an atom | atom structure diagram |. Web drawing atomic structures schoolrevision 58 subscribers subscribe 120 share save 19k views 9 years ago in this video i have used the example of sodium, simply because. It is total number of proton and neutron present in the nucleus of each atom of an element. When constructing a lewis diagram, keep in mind the octet rule, which refers to. Then play a game to test your ideas! Elements and atoms elements and atoms matter, elements, and atoms introduction to the atom atomic structure atomic number, atomic mass, and isotopes atomic structure google classroom what three particles make up an atom? An electron is a negatively charged particle. Web how to draw an atomic structure yokidz 344k subscribers subscribe 659. Hydrogen has 1 proton and no neutrons. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Of proton + no of neutrons. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Web draw your protons and neutrons. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.. It is the smallest of the main 3 particles that comprise an atom. Since protons are the same as the amount of electrons, you just draw 6 protons. Web drawing atomic structures schoolrevision 58 subscribers subscribe 120 share save 19k views 9 years ago in this video i have used the example of sodium, simply because. It is total number of proton and neutron present in the nucleus of each atom of an element. Web campus bookshelves smith college chm 222 chemistry ii: Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Web it also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Erase the c in the center circle, and draw in your protons. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. In the bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The mass number of fluorine is 19 and atomic number is 9. Electrons orbit the nucleus of an atom which is made up of both protons and neutrons. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic table tiles.
Simple model of atom structure with electrons vector image on VectorStock Electrons, Atom

How to Draw an Atom Really Easy Drawing Tutorial
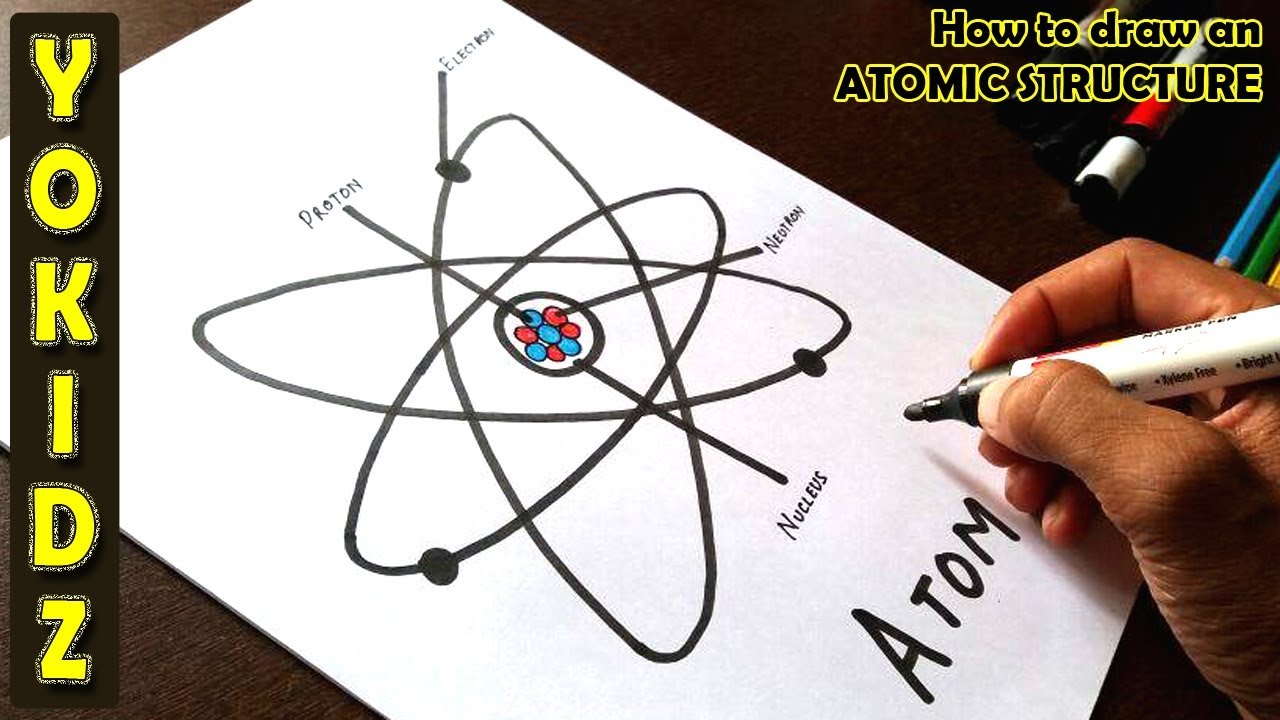
How to draw an ATOMIC structure YouTube
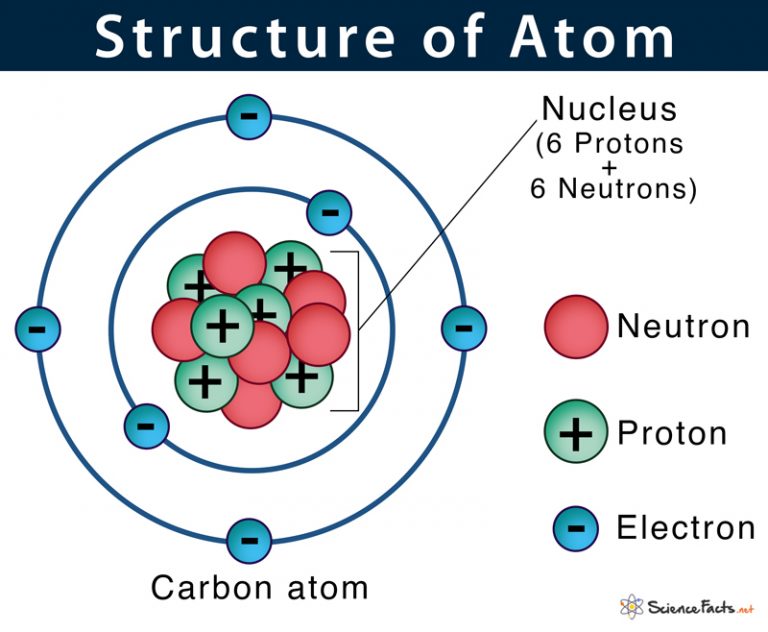
Atom Definition, Structure & Parts with Labeled Diagram
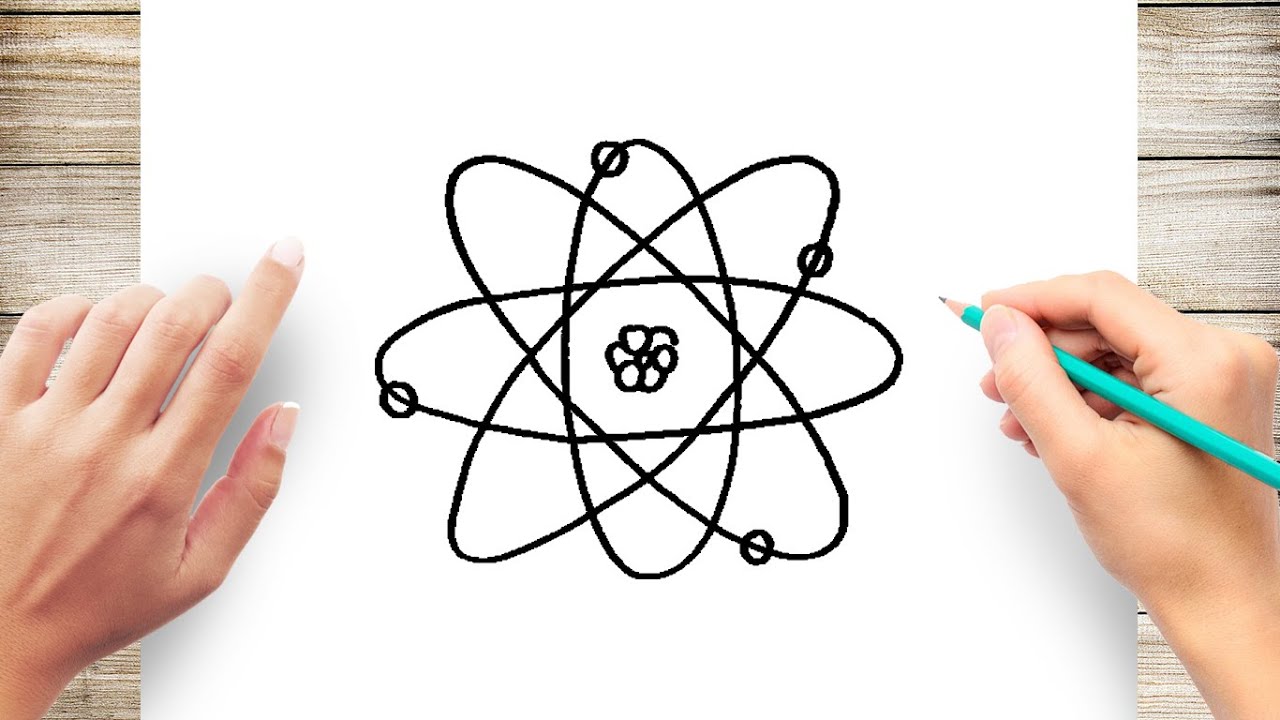
How to Draw an Atom Step by Step Simple and Easy YouTube
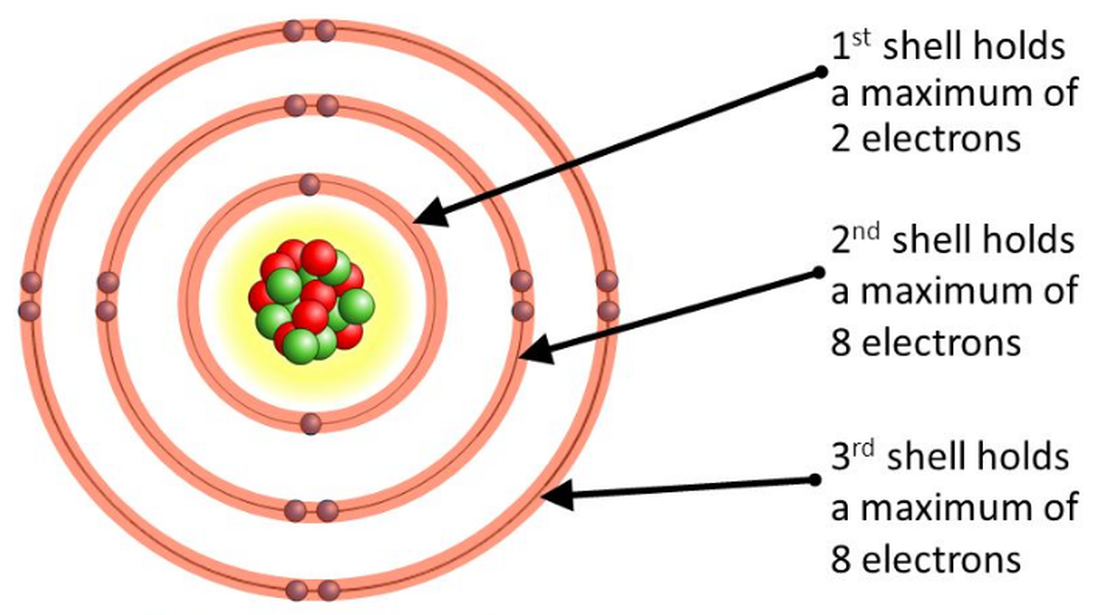
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

Atom Definition, Structure & Parts with Labeled Diagram

About Atomic Structure

How to Draw an Atom Really Easy Drawing Tutorial

Drawing Atoms Montessori Muddle
All Atom Labels Are Shown And All Lone Pairs Are Shown.
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As Dots Next To Atoms.
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
The Reason For Learning To Draw Lewis Structures Is To Predict The Number And Type Of Bonds That May Be Formed Around An Atom.
Related Post: