How To Draw Dipole Arrows
How To Draw Dipole Arrows - Web 11k views 8 years ago chemistry 1 dipole arrows are used anytime a molecule possesses a dipole moment, which happens when a molecule is polar. The more modern way to note a dipole moment is an arrow pointing towards the positive charge. Web 1 answer rzp7 · stefan v. And electric dipole moment vectors point from the negative to the positive charge. If these arrows, or the dipole moments cancel out, the molecule is nonpolar. Web dipole arrows are drawn pointing from positive to negative center. It explains how to indic. We start by looking at a water molecule: Using the cross bow arrow shown below we can show that it has a net dipole. Web the arrow refers to the direction of the dipole moment. Web the convention in chemistry is that the arrow representing the dipole moment goes from positive to negative. People keep using center here, and i think students will misinterpret this. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Postby lauren ton 4b ». Top eliana witham 2h posts: Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. For dipole arrows at an angle, separate them into horizontal and vertical vector components. Step 4) look at the dipoles. Web step 2) draw dipoles for each bond. Dipole arrows point towards the more electronegative element. Dipole arrows postby eliana witham 2h » mon nov 30, 2020 7:48 am dipole arrows represent the dipole moment in a molecule. When you draw a dipole moment, the old way of signifying the dipole moment was an arrow pointing towards the negative charge. The arrow points towards the more electronegative atom.. Web dipole moment arrows. Web the arrow refers to the direction of the dipole moment. Apr 5, 2017 looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: If a polar bond exists then dipole arrows must. If these arrows, or the dipole moments cancel out, the molecule is nonpolar. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Web what do they show? When you draw a dipole moment, the old way of signifying the dipole moment was an arrow pointing towards the negative charge. The more modern way to note a dipole moment is an arrow. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. Web draw in dipole arrows for all polar covalent bonds, starting the arrow at the more electropositive atom, and ending at the more electronegative atom. Web about press copyright contact us creators advertise developers terms privacy policy & safety how. Using a molecular geometry chart will let them view these as having positive and negative sides, and we draw the arrow toward the negative side. Web 1 answer rzp7 · stefan v. And electric dipole moment vectors point from the negative to the positive charge. It explains how to indic. A small plus sign is drawn on the less electronegative. Top eliana witham 2h posts: This is why in water the dipole arrows are drawn going from hydrogen (low electronegativity) towards oxygen (higher electronegativity). If these arrows, or the dipole moments cancel out, the molecule is nonpolar. A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density.. Web 28 i understand that molecular dipoles are electric dipoles. People keep using center here, and i think students will misinterpret this. Web the convention in chemistry is that the arrow representing the dipole moment goes from positive to negative. If these arrows, or the dipole moments cancel out, the molecule is nonpolar. Also learn how to draw a dipole. Thu oct 01, 2020 4:48 am been upvoted: Postby lauren ton 4b » mon nov 03, 2014 4:00 am. In class we learned to draw these special molecular dipole arrows (with a plus at the beginning) that point from the positive to the negative partial charge. Web what do they show? Web this chemistry video tutorial provides a basic introduction. Web 1 answer rzp7 · stefan v. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. For dipole arrows at an angle, separate them into horizontal and vertical vector components. Web because of this, the polarization of covalent bonds is typically shown using a special arrow (a dipole arrow) to indicate the direction in which the bond is polarized. For dipole arrows at an angle, separate them into horizontal and vertical vector components. The net dipole is the measurable, which is called the dipole. Physicist tend to use the opposite orientation. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. Dipole arrows point towards the more electronegative element. This is why in water the dipole arrows are drawn going from hydrogen (low electronegativity) towards oxygen (higher electronegativity). Web what do they show? A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density. In class we learned to draw these special molecular dipole arrows (with a plus at the beginning) that point from the positive to the negative partial charge. People keep using center here, and i think students will misinterpret this.[Solved] how to draw the net dipole arrows for NF3, and which ones are

How Do You Determine Which Way the Dipole Arrow Points

Drawing dipole arrows and determining molecular polarity YouTube

Understanding Dipole Arrows YouTube
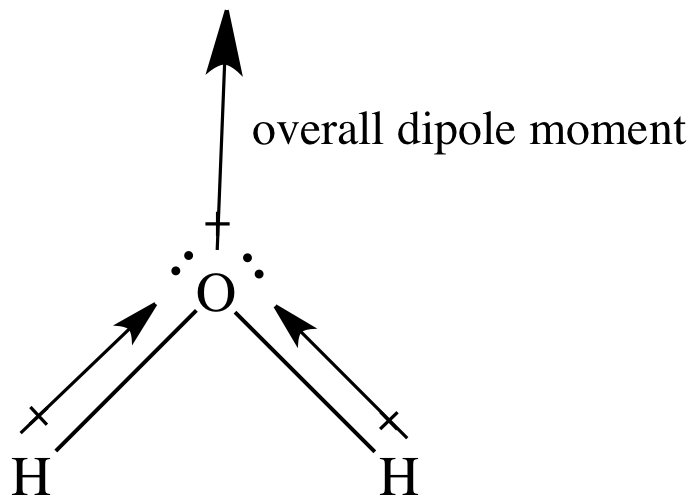
In a sketch of the bent watermolecule, the head of the two bond dipole

How To Draw Overall Dipole Moment DRAWINGS OF LOVE
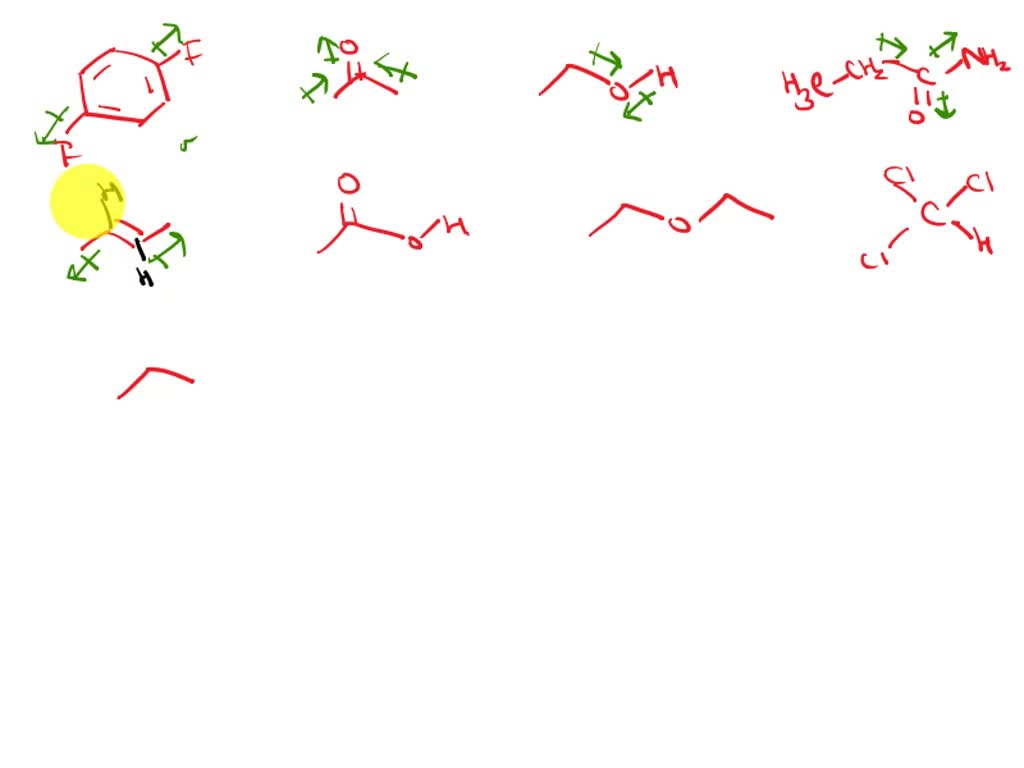
SOLVED Draw Dipole arrows for all of the polar covalent bonds in the
[Solved] how to draw the net dipole arrows for H2S, and which ones are
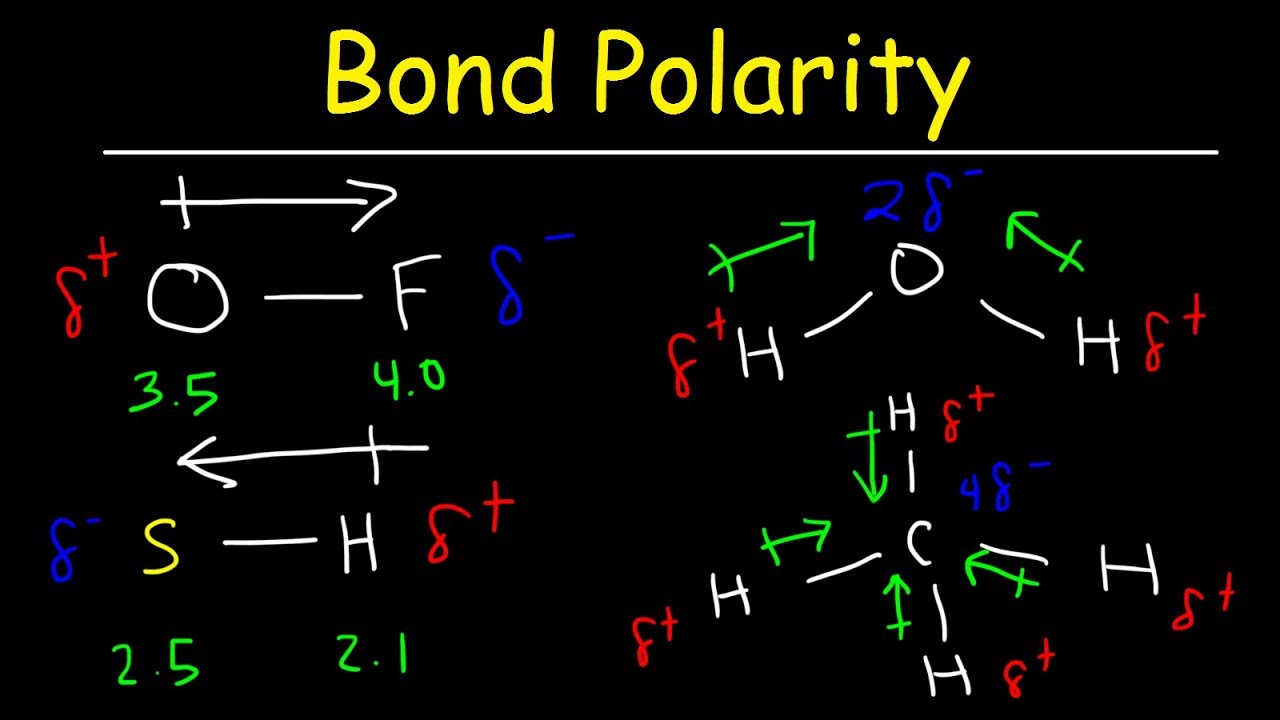
Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice
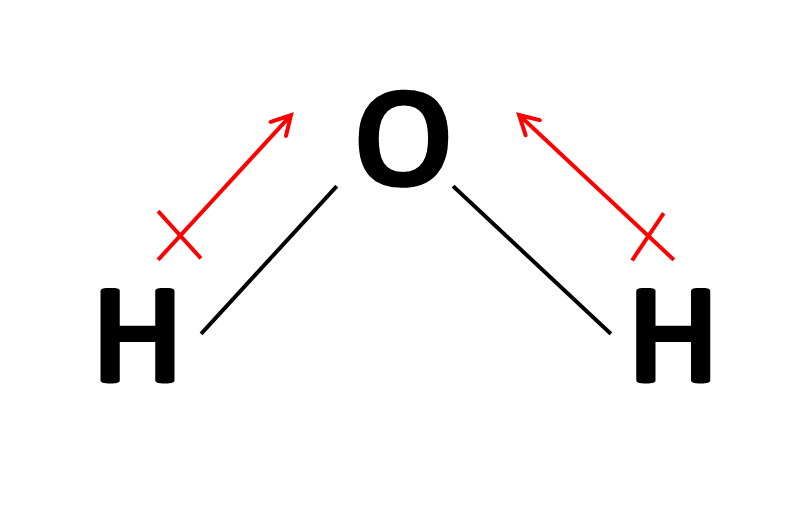
How do we draw the dipole moment of a water molecule? Socratic
Web The Convention In Chemistry Is That The Arrow Representing The Dipole Moment Goes From Positive To Negative.
Apr 5, 2017 Looking At The Electronegativity And Shape Of The H2O Molecule Tells You How The Arrow Depicts The Polarity:
Web Step 2) Draw Dipoles For Each Bond.
If A Polar Bond Exists Then Dipole Arrows Must.
Related Post: