How To Draw Hydrogen Atom
How To Draw Hydrogen Atom - Web (hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. Web an exception is that hydrogen is almost never a central atom. E ( n) = − 1 n 2 ⋅ 13.6 ev In general we try to get the octet rule for any atom except for hydrogen. Web describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum; Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. Web the hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. Web steps to draw the lewis structure of h2 step #1: We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h. Calculate the total number of valence electrons. Each carbon atom has four bonds. Make sure the shape is correct. Either draw a line diagram or show every single atom and bond. Chains of carbon atoms joined by single bonds should be kinked. The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move). Either draw a line diagram or show every single atom and bond. Web an exception is that hydrogen is almost never a central atom. Hydrogen. The radius of the first bohr orbit is called the bohr radius of hydrogen, denoted as a0. Web steps to draw the lewis structure of h2 step #1: It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. But fluorine, you want to get it to eight. Web key points bohr's model of hydrogen is. Identify the physical significance of each of the quantum numbers (n, l, m) of the hydrogen atom; Each nitrogen atom has three bonds. As the most electronegative element, fluorine also cannot be a central atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. It results in the emission of. Web instead of a literal ionized single hydrogen atom being formed, the acid transfers the hydrogen to h 2 o, forming h 3 o +. Web so now, our general rule of thumb would be, try to put those on those terminal atoms with the goal of getting those terminal atoms to having eight valence electrons. This is shown in. Distinguish between the bohr and schrödinger models of the atom This is shown in the two models below. If instead a hydrogen atom gains a second electron, it becomes an anion. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system. Add enough electrons (dots) to the outer atoms. Either draw a line diagram or show every single atom and bond. The only difference between these is a slight rotation of the bond between the centre two carbon atoms. E ( n) = − 1 n 2 ⋅ 13.6 ev Make sure the shape is correct. Web instead of a literal ionized single hydrogen atom being formed, the acid. Web instead of a literal ionized single hydrogen atom being formed, the acid transfers the hydrogen to h 2 o, forming h 3 o +. Its value is obtained by setting n = 1 in equation 6.5.6: So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Distinguish between the bohr and schrödinger models of the atom Each oxygen atom has two bonds. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of.. Web always draw hydrogen atoms attached to any heteroatom (an atom that is no carbon) on both structural and line diagrams. Hydrogen, you just need to get to two in that outer shell. The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move). A very simple. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from. Each oxygen atom has two bonds. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web in this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Web instead of a literal ionized single hydrogen atom being formed, the acid transfers the hydrogen to h 2 o, forming h 3 o +. Bohr's model calculated the following energies for an electron in the shell, n : Chains of carbon atoms joined by single bonds should be kinked. Each hydrogen atom has one bond. Make sure the shape is correct. Web describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum; In order to draw the lewis structure of h2, first of all you have to find the total number of valence electrons present in the h2 molecule. Web step 1: Calculate the total number of valence electrons. This is shown in the two models below. I show you where hydrogen is on the periodic table and how to determine how many valence electrons it has.
Hydrogen Atom Diagram

Describe Bohr’s model of the hydrogen atom. bitWise Academy
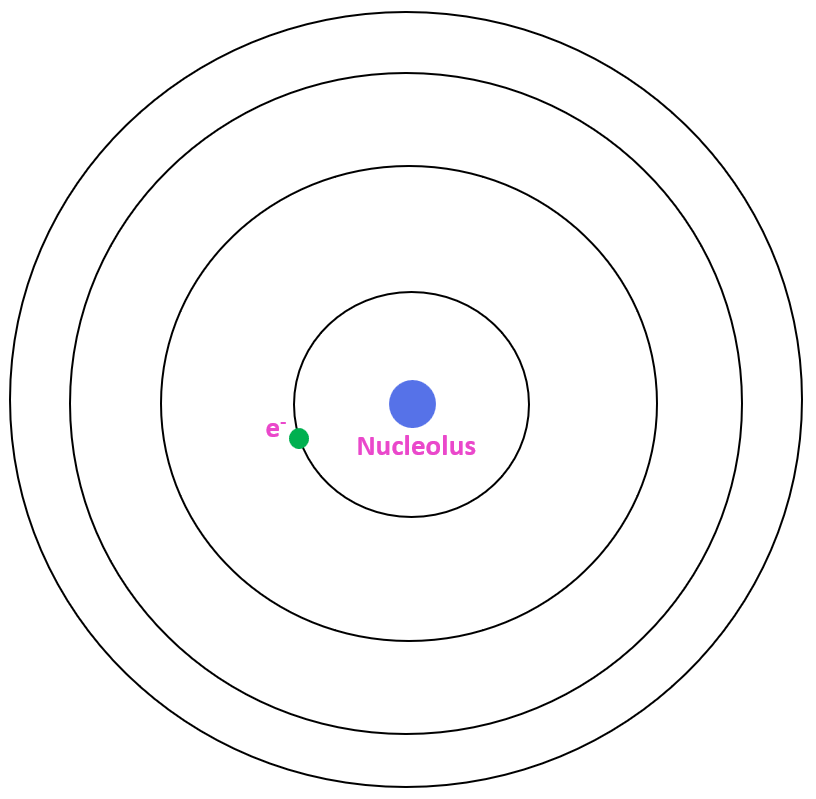
Bohr Model of the Hydrogen Atom Chemistry Steps

Diagram Representation Of The Element Hydrogen Stock Vector Image

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
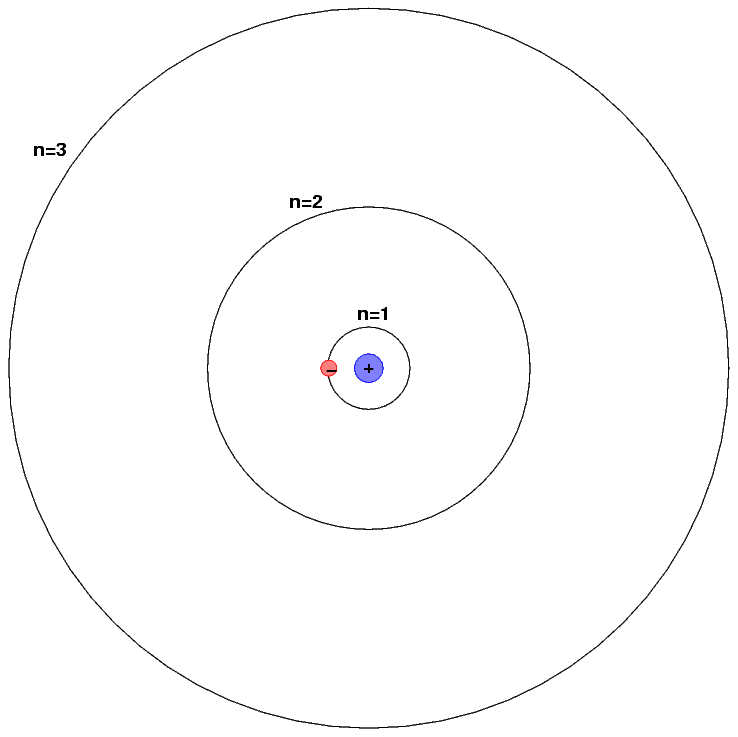
GJ Blogs the atomic structure of hydrogen

Diagram representation element hydrogen Royalty Free Vector

Hydrogen atom on white background Royalty Free Vector Image

Hydrogen Definition, Structure, Properties & Uses Embibe

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
Web And To Draw The Atoms You Fill Up The Inner Shells First Then Move On To The Outer Shells.
In General We Try To Get The Octet Rule For Any Atom Except For Hydrogen.
Thus The Most Probable Radius Obtained From Quantum Mechanics Is Identical To The Radius Calculated By Classical Mechanics.
Web Using Conventional Bond Notation, You Could Draw It As, For Example:
Related Post: