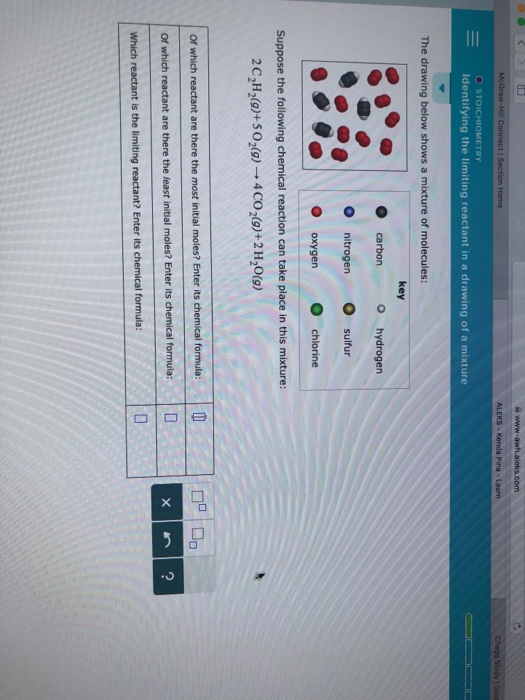
Identifying The Limiting Reactant In A Drawing Of A Mixture
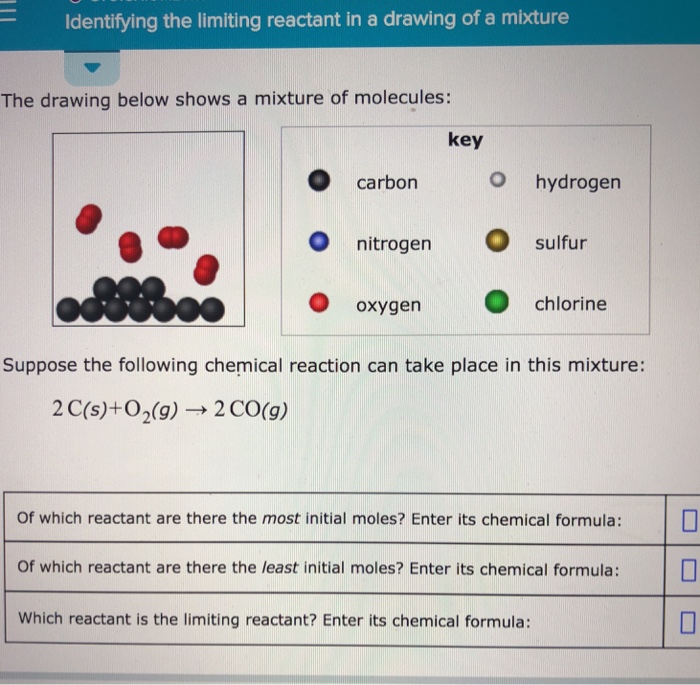
Identifying The Limiting Reactant In A Drawing Of A Mixture - Web the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The limiting reactant is rb since it would yield the least amount of product (0.711 g mg). Calculate the mass of excess reactant that reacts. (g)+2o2 (g) → co2 (g)+2h2o (g) of which reactant are there the most initial moles? To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation. O chemical reactions identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: 4 this problem has been solved! Web identifying the limiting reactant in a mixture of molecules: Convert all given information into moles. Web as we saw in example 1, there are many different ways to determine the limiting reactant, but they all involve using mole ratios from the balanced chemical equation. How to solve a tricky version of the aleks “limiting reactant” problem. Count the number of particles in the drawing given. Carbon nitrogen oxygen key o hydrogen sulfur chlorine suppose the following chemical reaction can take place in this mixture: Web identifying the limiting reactant in a mixture of molecules: Cs₂(g)+30₂(g) → co₂(g) +2 so₂(g) of which reactant are there. Calculate the mole ratio from the given information. Web try it free. Web = o chemical reactions identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: What we need to do is determine an amount of one product (either moles or mass) assuming all of each reactant reacts. Web identifying the. (g)+2o2 (g) → co2 (g)+2h2o (g) of which reactant are there the most initial moles? 2c2h2 (g)+so2 (g)_+4co2 (9) + 2h2og) of which reactant are there. \begin{tabular}{ll} \hline key & hydrogen \\ nitrogen & sulfur \\ oxygen & chlorine \\ \hline \end{tabular} suppose the following chemical reaction can take place in this mixture: Determine which substance will run. Stoichiometry identifying. To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation. O o carbon nitrogen oxygen key o hydrogen o sulfur chlorine suppose the following chemical reaction can take place in this mixture: Stoichiometry identifying the limiting reactant in a drawing. Web chemical reactions identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: To identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the balanced chemical equation. Look at the balanced reaction and determine how many. Count the number of particles in the drawing given. Web determining the limiting reactant. The excess reactant is mgcl 2 since its complete reaction would have yielded up to 0.878 g mg. O o carbon nitrogen oxygen key o hydrogen o sulfur chlorine suppose the following chemical reaction can take place in this mixture: Web about press copyright contact us. Determine which substance will run. O 0 carbon nitrogen oxygen key o hydrogen. Web enter its chemical formula: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which reactant is the limiting reactant? Stoichiometry 一 identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules key o carbon o hydrogen o nitrogen sulfur o oxygenchlorine suppose the following chemical reaction can take place in this mixture: 2c2h2 (g)+so2 (g)_+4co2 (9) + 2h2og) of which reactant are there. Web chemical reactions identifying the limiting reactant in. Web identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules: \begin{tabular}{ll} \hline key & hydrogen \\ nitrogen & sulfur \\ oxygen & chlorine \\ \hline \end{tabular} suppose the following chemical reaction can take place in this mixture: Web chemical reactions identifying the limiting reactant in a drawing of a mixture the. 2 so₂ (g) + o₂ (g) → 2 so 3 (g) of which reactant are there the most initial. Stoichiometry 一 identifying the limiting reactant in a drawing of a mixture the drawing below shows a mixture of molecules key o carbon o hydrogen o nitrogen sulfur o oxygenchlorine suppose the following chemical reaction can take place in this mixture:. \begin{tabular}{ll} \hline key & hydrogen \\ nitrogen & sulfur \\ oxygen & chlorine \\ \hline \end{tabular} suppose the following chemical reaction can take place in this mixture: O 0 carbon nitrogen oxygen key o hydrogen. Web how to identify the limiting reactant (limiting reagent) example \(\pageindex{1}\): Determine which substance will run. Count the number of particles in the drawing given. Carbon nitrogen oxygen key o hydrogen sulfur chlorine suppose the following chemical reaction can take place in this mixture: Suppose the following chemical reaction can take place in this mixture: Key o hydrogen carbon nitrogen sulfur oxygen chlorine suppose the following chemical reaction can take place in this mixture: In the real world, amounts of reactants and products are typically measured by mass or by volume. Cs₂(g)+30₂(g) → co₂(g) +2 so₂(g) of which reactant are there the most initial. Your answer is incorrect the drawing below shows a mixture of molecules: Web high school chemistry skills practice 1. (g)+2o2 (g) → co2 (g)+2h2o (g) of which reactant are there the most initial moles? Ch (9)+20,(9) + co2(9)+2h 0(0) of which reactant are there the most initial moles? Web try it free. Compare the calculated ratio to the actual ratio.
ALEKS Identifying the Limiting Reactant in a Drawing of a Mixture
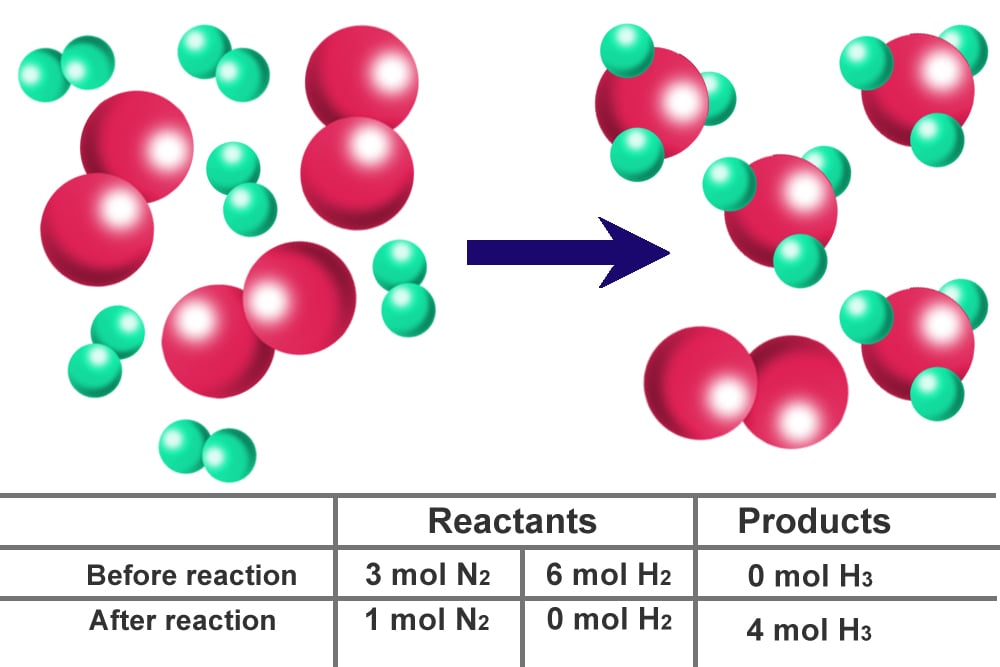
How To Find The Limiting Reactant In A Chemical Reaction?
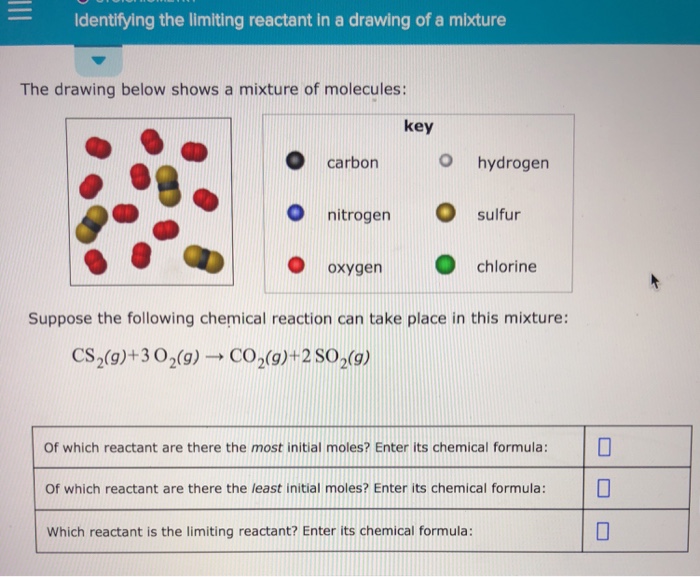
(Solved) Identifying The Limiting Reactant In A Drawing Of A Mixture
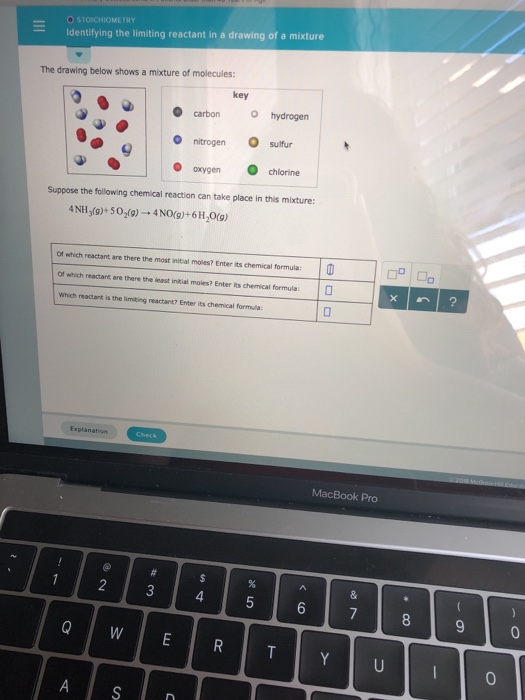
(Get Answer) STOICHIOMETRY ? Identifying The Limiting Reactant In A
Solved O STOICHIOMETRY Identifying The Limiting Reactant

ALEKS Identifying the Limiting Reactant in a Drawing of a Mixture
(Solved) Identifying The Limiting Reactant In A Drawing Of A Mixture

5.3b Identifying the limiting reactant in a drawing of a mixture YouTube

Aleks Identifying the limiting reactant in a drawing of a mixture YouTube
How to Identify the Limiting Reactant in a Drawing of a Mixture
Which Reactant Is The Limiting Reactant?
2 So₂ (G) + O₂ (G) → 2 So 3 (G) Of Which Reactant Are There The Most Initial.
The Limiting Reactant Is Rb Since It Would Yield The Least Amount Of Product (0.711 G Mg).
Ch4(G)+2O2(G)→Co2(G)+2H2O(G) Of Which Reactant Are There.
Related Post: