Specific Heat Drawing
Specific Heat Drawing - Web this chemical property, known as specific heat, is defined as the amount of thermal energy needed to raise the temperature of 1 gram of a substance by 1 degree celsius. Web by following these steps and standards, you can create a mechanical drawing with specific heat treatment requirements, that conveys your design intent and specifications clearly and. Q = \(mc\delta t\) derivation of specific heat formula The choice is yours 路 ♂️ most of us have either been there or still stuck there. 1.00 cal = 4.184 j specific heat (c s): Think about your question then watch the video again and you will see that sal is reenforcing your point using generalized intuition as opposed to specifics. Also, the formula is like this: There are reasons he teaches this way and they are all good. Namely, by measuring the heat capacity of a sample of the substance, usually with a calorimeter, and dividing by the sample's mass. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00ºc. C 2 h 6 o(l) 2.376: The amount of heat absorbed or released by a substance depends directly on the type of substance, its mass, and the. Web specific heat and heat capacity are measures of the energy needed to change the temperature of a substance or object. The units of specific heat are usually calories or joules per gram. Substance symbol (state) specific heat (j/g °c) helium: Web the specific heat of aluminum is 903 j/kg•k. Specific heat is temperature (and phase) dependent. Web specific heat 8/4/2018 2revised joule (j): Unit of heat most commonly used in the si system. The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00 c. Its si unit is j/ (kg ⋅ ⋅ k) or j/ (kg ⋅ ⋅ °c °c ). Q q is the amount of supplied or subtracted heat (in joules), m m is the mass of the sample, and \delta. 1.00 cal = 4.184 j specific heat (c s): Web sal was explaining the intuition behind the formula for thermal conductivity, he was not solving for specific heat ratios. The amount of heat absorbed or released by a substance depends directly on the type of substance, its mass, and the. The temperature change ( δt δ t) is the same. Where q is the heat gained or lost, m is the mass of the object, c is its specific heat, and δt is the change in. The amount of heat gained or lost by an object when its temperature changes can be calculated by the formula. Web the specific heat is the amount of heat necessary to change the temperature. The equation that relates heat (q) ( q) to specific heat (cp) ( c p), mass (m) ( m), and temperature change (δt) ( δ t) is shown below. Metals and semimetals common liquids and fluids c) = 5/9 [t ( c)] (9/5) + 32 the energy required to heat a product can be calculated as m dt (1) q. The amount of heat gained or lost by an object when its temperature changes can be calculated by the formula. The temperature change ( δt δ t) is the same in units of kelvins and degrees celsius (but not degrees fahrenheit). Web the symbol c stands for specific heat and depends on the material and phase. Web this chemical property,. Heat capacity and specific heat The specific heat c is a property of the substance; Web by following these steps and standards, you can create a mechanical drawing with specific heat treatment requirements, that conveys your design intent and specifications clearly and. The specific heat c is a property of the. Web specific heat, the quantity of heat required to. The equation that relates heat (q) ( q) to specific heat (cp) ( c p), mass (m) ( m), and temperature change (δt) ( δ t) is shown below. There are reasons he teaches this way and they are all good. Heat capacity and specific heat Metals and semimetals common liquids and fluids c) = 5/9 [t ( c)] (9/5). Metals and semimetals common liquids and fluids c) = 5/9 [t ( c)] (9/5) + 32 the energy required to heat a product can be calculated as m dt (1) q = heat required (kj) = specific heat (kj/kg k, kj/kg dt = temperature difference (k, Also, the formula is like this: Its si unit is j/ (kg ⋅ ⋅. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Web the specific heat capacity of a substance is typically determined according to the definition; Heat capacity and specific heat H 2 o(s) 2.093 (at −10 °c) water vapor: Q = \(mc\delta t\) derivation of specific heat formula The equation that relates heat (q) ( q) to specific heat (cp) ( c p), mass (m) ( m), and temperature change (δt) ( δ t) is shown below. Web specific heat capacity (often just called specific heat) is the amount of heat energy (usually in joules) necessary to increase the temperature of one gram of substance by one degree celsius or one kelvin. Web specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one celsius degree. Web specific heat online unit converter see also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. Specific heat is temperature (and phase) dependent. (recall that the temperature change δt is the same in units of kelvin and degrees celsius.) values of specific heat must generally be measured, because there. Web the si unit for specific heat is j / (kg × k) or j / (kg ×oc). Q q is the amount of supplied or subtracted heat (in joules), m m is the mass of the sample, and \delta t δt is the difference between the initial and final temperatures. Where q is the heat gained or lost, m is the mass of the object, c is its specific heat, and δt is the change in. Web the formula for specific heat looks like this: Web specific heat online unit converter.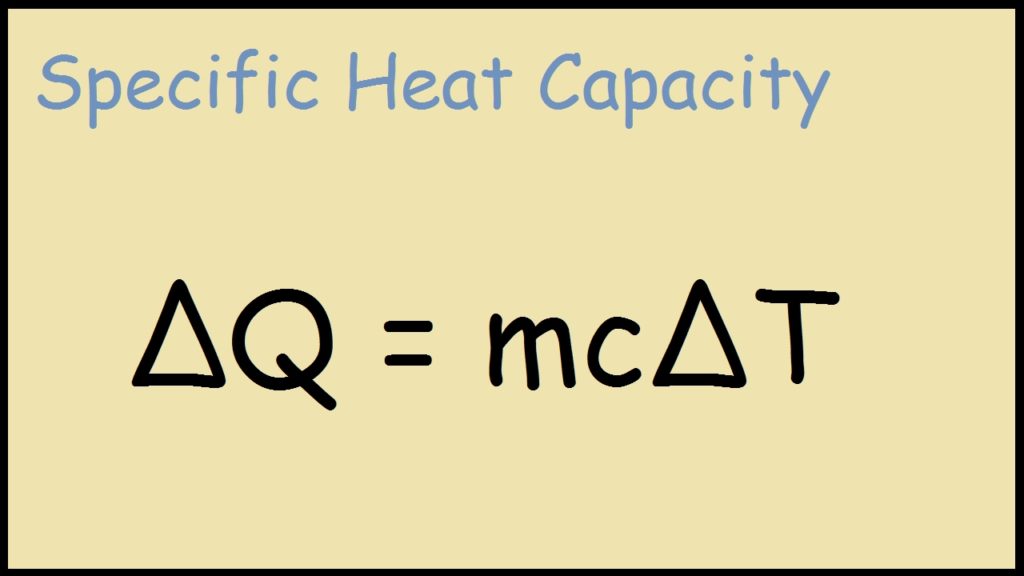
Specific Heat Formula Definition, Equations, Examples
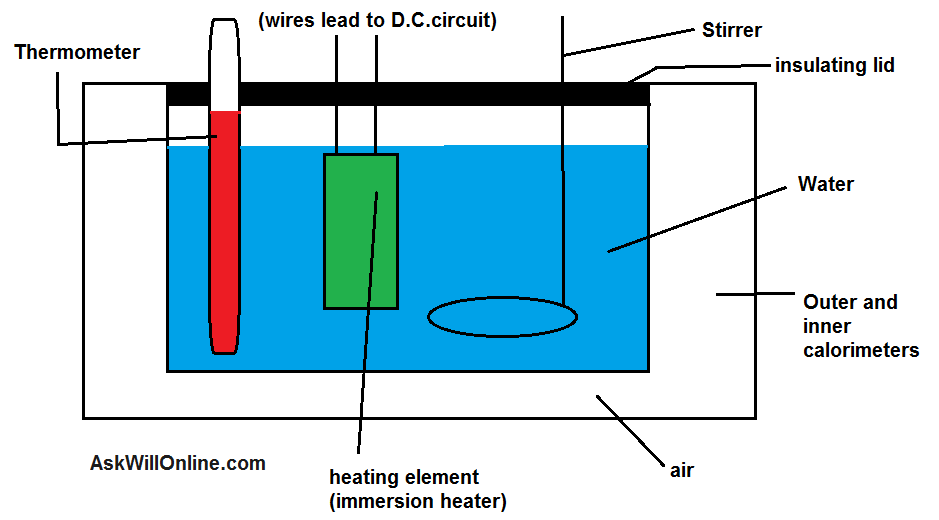
Specific Heat Capacity and Latent Heat Experiments In Physics Ask
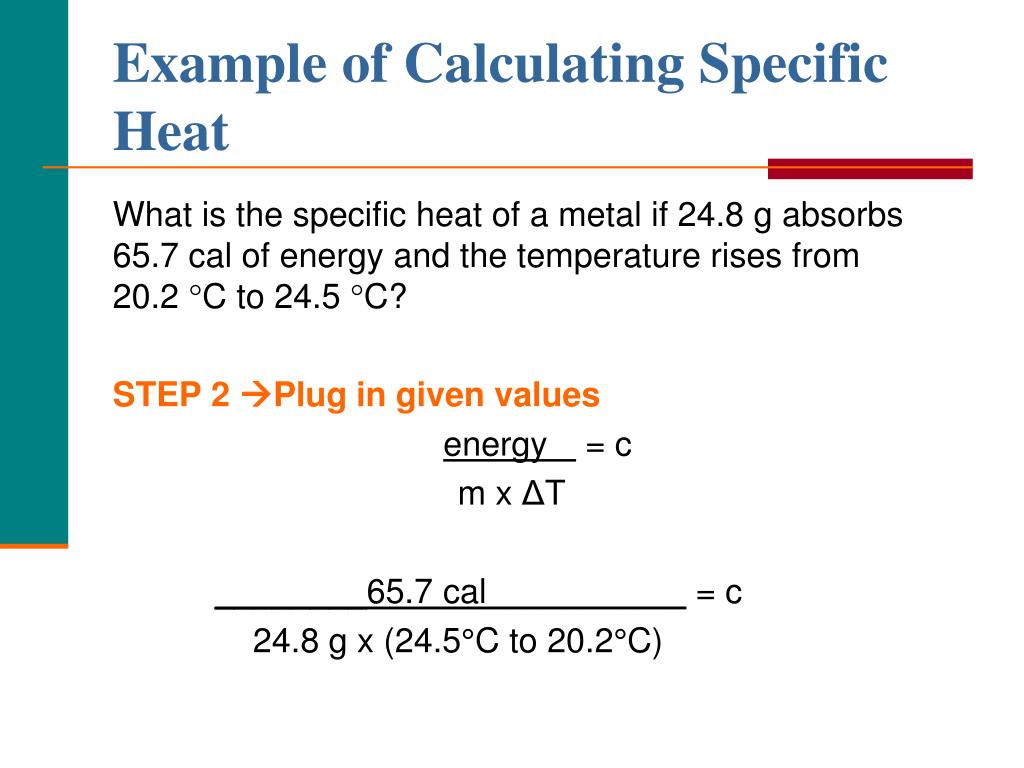
How To Calculate Specific Heat Of Metal Haiper
Lesson Video Specific Heat Capacity Nagwa

AQA A Level Physics Year 2 /IB Physics Specific Heat Capacity
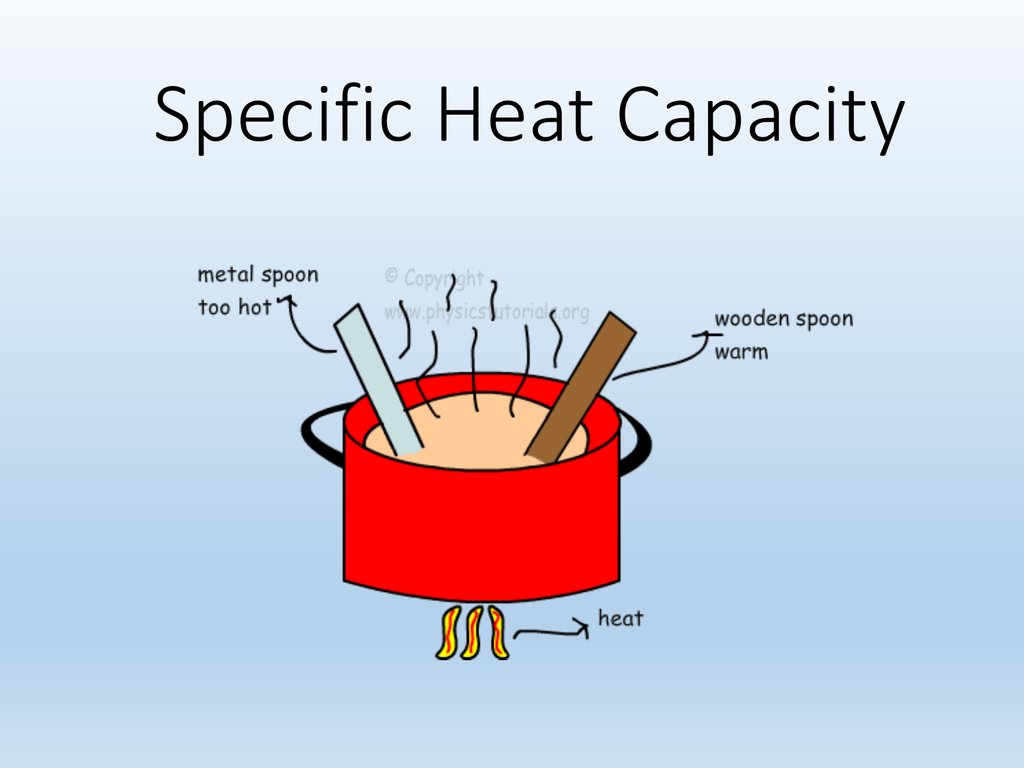
1.3 Specific Heat Capacity
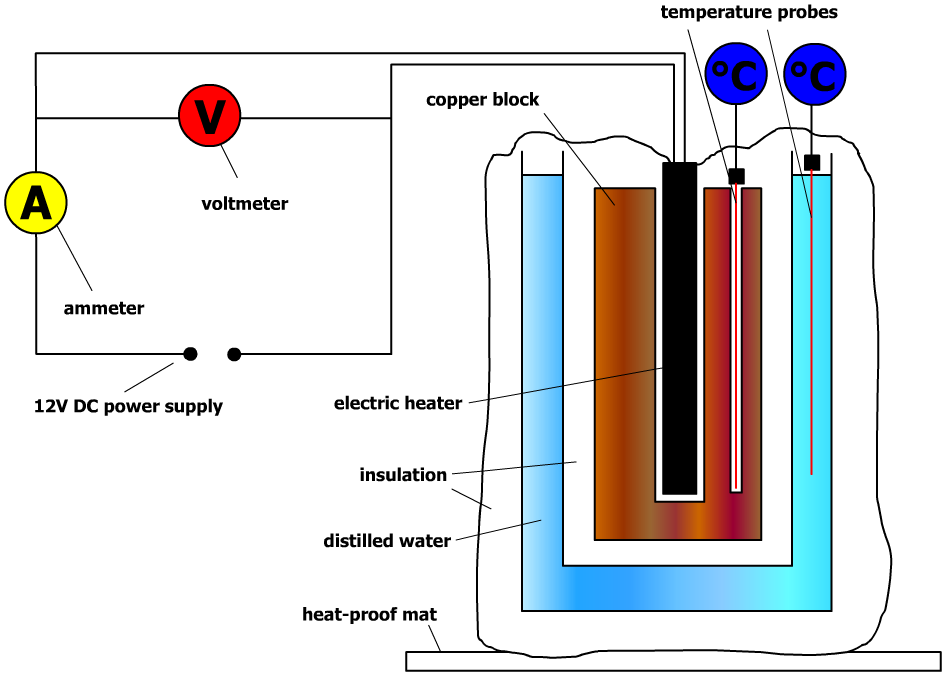
Finding a material's specific heat capacity GCSE Science Marked by

Lesson 10 Specific Heat YouTube
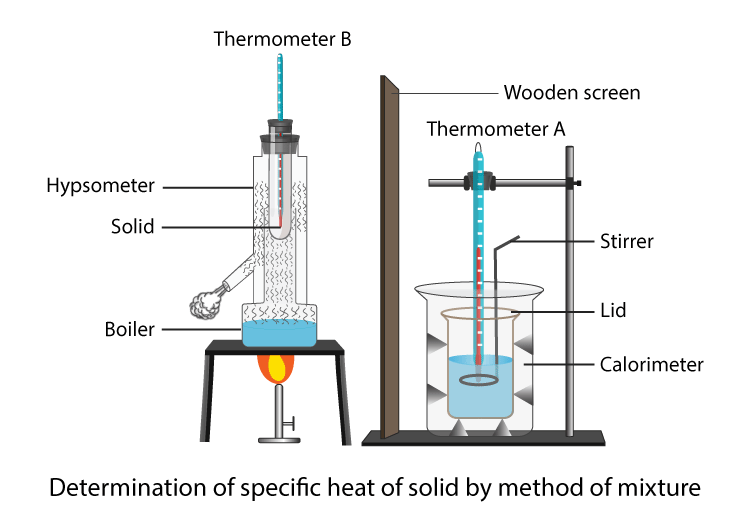
To Determine Specific Heat Capacity Of A Given Solid Physics Practical
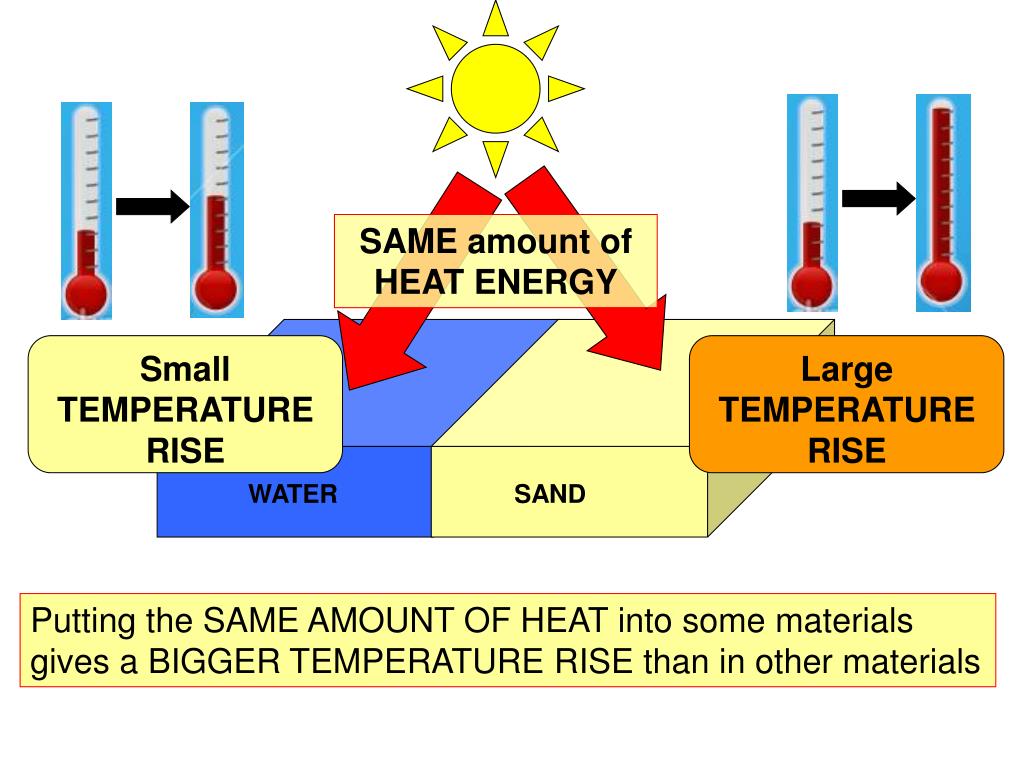
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download
As We Discussed Above The Specific Heat Is The Relation Of Temperature Change Of An Object With Water.
C 2 H 6 O(L) 2.376:
The Units Of Specific Heat Are Usually Calories Or Joules Per Gram Per Celsius Degree.
Think About Your Question Then Watch The Video Again And You Will See That Sal Is Reenforcing Your Point Using Generalized Intuition As Opposed To Specifics.
Related Post: